Why first-void urine could potentially change the future of HPV screening
Posted: 12 November 2025 | Drug Target Review | No comments yet
From richer biomarker content to patient-friendly sampling, first-void urine is emerging as a promising tool in precision health. Here is why scientists are paying attention.

Human papillomavirus (HPV) is one of the most common sexually transmitted infections worldwide that leads to the development of cervical cancer. Screening programmes have been highly effective in reducing incidence and mortality, yet accessibility remains a challenge, with cultural, logistical and emotional barriers preventing many individuals from attending clinical appointments or undergoing invasive procedures.
One approach attracting increased attention is the use of first-void urine (FVU) – the very first portion of urine released at the start of urination. Unlike mid-stream urine, which is more commonly collected for urinary tract infections, FVU is enriched with epithelial cells, nucleic acids and other biomarkers from the lower urinary tract. These characteristics make it a potentially promising sample type for non-invasive collection in both infectious disease and oncology fields. It is also gaining attention in drug discovery, where early and accessible biomarkers can inform translational research and precision medicine strategies.
To understand the science and potential of FVU, we spoke with Tara Crawford Parks, PhD, Director of Translational R&D and Product Enablement at DNA Genotek™. With a PhD in RNA binding proteins and a career dedicated to translational medicine, Tara now leads initiatives that connect fundamental research with practical applications in precision health.
Automation now plays a central role in discovery. From self-driving laboratories to real-time bioprocessing
This report explores how data-driven systems improve reproducibility, speed decisions and make scale achievable across research and development.
Inside the report:
- Advance discovery through miniaturised, high-throughput and animal-free systems
- Integrate AI, robotics and analytics to speed decision-making
- Streamline cell therapy and bioprocess QC for scale and compliance
- And more!
This report unlocks perspectives that show how automation is changing the scale and quality of discovery. The result is faster insight, stronger data and better science – access your free copy today
Distinct characteristics of first-void urine
Traditional urine collection relies on mid-stream urine (MSU), where the initial flow is discarded, for the purpose of reducing biomarkers from the genitals and lower urinary tract; however, this practice also removes the biologically relevant material for other applications.
First-void urine, also referred to as first catch or first pass urine, is the first fraction of urine produced, up to the first 50mL of urine, that exhibits elevated concentrations of biomarkers.
As Tara explained, “First-void urine, also referred to as first catch or first pass urine, is the first fraction of urine produced, up to the first 50mL of urine, that exhibits elevated concentrations of biomarkers.”
FVU contains epithelial cells, mucus, extracellular vesicles, DNA, RNA, proteins and metabolites originating from the urethra and lower urinary tract. These materials can be analysed in molecular assays to detect infection, inflammation or early signs of disease. For researchers, this also provides a non-invasive window into molecular changes that could inform drug target discovery and therapeutic response.
The World Health Organization (WHO) already recommends FVU as the preferred sample type for nucleic acid amplification tests for Chlamydia trachomatis and Neisseria gonorrhoeae, but its biomarker richness positions it for other potential screening uses, including HPV detection and oncology research.
How the Colli-Pee™ device standardises first-void urine collection
Accurately collecting FVU with a urine cup is challenging. Given that the requisite diagnostic material is concentrated in the very first fraction of urine, there is a high risk of losing this volume or else diluting the sample.
To address this challenge, purpose-designed devices have been developed. “The Colli-Pee™ device standardises first-void urine collection by channelling urine into the collection tube until the designated volume is collected, at which point the float rises, redirecting the remainder of the urine through the device and into the toilet,” Tara noted.
This mechanism ensures consistent volumes across different device formats (4mL, 10mL, 20mL and 40mL). Internal studies have shown that these collections are far more reproducible than cup-based methods. For participants, the process is discreet, simple and well suited to self-collection outside of clinical settings.
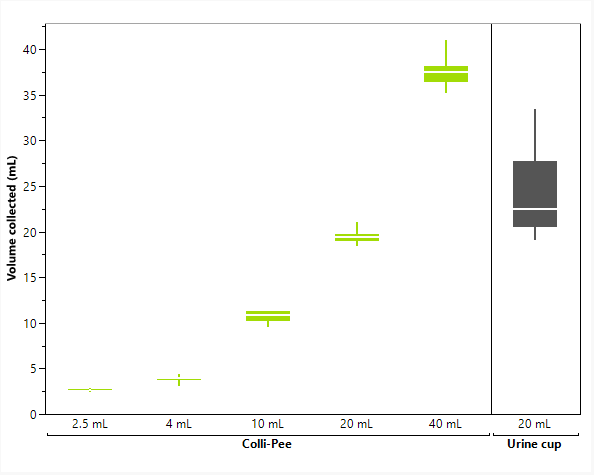

Comparison of first-void urine volumes collected with the Colli-Pee™ device versus standard urine cups. The Colli-Pee™ device demonstrates consistent volumetric sampling across formats, while urine cups show greater variability.
Tara emphasised that this standardisation is critical because it guarantees reliable material for downstream analysis – something that has been difficult to achieve with conventional approaches.
Beyond HPV
FVU contains a range of molecules that make it useful for multiple areas of research. “First-void urine can contain a wide array of analytes including cell-free or cellular DNA and RNA, extracellular vesicles, proteins and metabolites,” she said.
This spectrum of biomarkers opens possibilities for:
- Genomics and epigenomics – analysis of inherited variation or methylation changes linked to disease.
- Transcriptomics – RNA profiles associated with infection or cancer.
- Proteomics and metabolomics – protein and metabolite signatures of metabolic or inflammatory states.
- Oncology – early detection of prostate, breast or endometrial cancer, as well as monitoring recurrence.
- Women’s health – potential applications in conditions such as endometriosis.
Tara highlighted that the diversity of analytes supports both screening and long-term monitoring through a single, non-invasive sample type. Such breadth can also potentially support drug discovery, where multiomic data can be leveraged to explore disease mechanisms and validate candidate biomarkers.
Stabilising DNA with UCM preservative
Like other biological materials, urine begins to degrade soon after collection. DNA and RNA are particularly vulnerable, and microbial activity can alter the composition of the sample. Traditional methods rely on rapid processing or cold-chain transport, both of which increase cost and complexity.
The Colli-Pee™ UCM preservative was thus developed to overcome these challenges. “The Colli-Pee™ UCM device is capable of stabilising DNA in urine, such as HPV, for research applications for seven days at room temperature,” she explained.
By mixing urine with preservative at the point of collection, integrity is maintained without refrigeration. Tara observed that this reduces logistical barriers, simplifies workflows and allows studies to be scaled more effectively; particularly in areas where cold-chain transport is impractical. In a drug discovery context, this reliability means samples can be collected consistently across sites and populations, strengthening clinical trial data.
Detecting HPV on molecular platforms
HPV detection is one of the most clinically relevant applications for FVU. The sample contains enriched viral DNA, which allows it to be processed on a wide range of molecular platforms with high sensitivity.
Emerging data in multiple scientific publications from Europe and the UK support non-inferiority to the standard of care samples for HPV detection.
“Emerging data in multiple scientific publications from Europe and the UK support non-inferiority to the standard of care samples for HPV detection,” Tara noted.
Trials including VALHUDES (Validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples), Predictors 5.1 (a randomised comparison of self-collected vaginal devices and urine for HPV testing), CoCoss (Concurrent Comparison of Self-Sampling Devices for HPV Detection) and the CASUS study (Belgian home-based urinary HPV self-sampling and acceptability) have compared first-void urine or self-collected samples with clinician-collected cervical samples. Across these studies, urine-based hrHPV testing showed non-inferior clinical performance and higher acceptability to participants.
Patient preference and acceptability
For screening programmes to succeed, accuracy must be matched by patient acceptability. Invasive procedures can deter participation, particularly for individuals with specific cultural concerns, prior trauma or discomfort with clinical settings.
“Other sample types, such as clinically collected cervical samples for HPV screening, can be very invasive, require a clinical visit and may have lower acceptability in patients with cultural or religious concerns, or [who] may have experienced past trauma,” she stressed.
Evidence supports a strong preference for FVU. In the CASUS study (Belgian home-based urinary HPV self-sampling and acceptability) two-thirds of female participants reported preferring home-based FVU self-sampling with the Colli-Pee™ device over physician-collected cervical samples. More than 96 percent found the device easy to use and said they would use it again.
Tara added that self-collection reduces discomfort, avoids travel and increases inclusivity, particularly for groups who are less likely to engage with traditional healthcare.
Expanding access through non-invasive sampling
The potential public health benefits of FVU are significant. Self-collection can:
- Reduce psychological and physical barriers – especially for trauma survivors, individuals with disabilities or those with cultural sensitivities.
- Improve access for underserved populations by removing the need for travel, childcare or time off work.
- Empower patient autonomy – giving individuals greater control over their health data and collection process.
- Simplify logistics and reduce costs – through room-temperature preservation and transport.
Tara reflected that these advantages make FVU testing more than a technical innovation – they position it as a tool for addressing inequities in screening.
For drug discovery, greater inclusivity in sampling also expands the diversity of data available for biomarker research and clinical development.
Conclusion
The future of screening depends on approaches that are both scientifically robust and acceptable to patients. First-void urine, standardised and stabilised through devices such as the Colli-Pee™ device, fulfils both of these requirements.
With strong evidence of non-inferiority to established methods, clear patient preference and potential applications extending beyond HPV to oncology and women’s health, FVU represents a new direction for diagnostics.
As Tara emphasised, “Non-invasive sampling methods – like first-void urine – have growing evidence to support how they can remove barriers to screening and as such increase accessibility.”
By reducing disparities, empowering patients and simplifying workflows, FVU could help make diagnostic medicine more accessible, effective and patient-focused. For drug discovery researchers, it also highlights how innovations in sample collection can open new possibilities for biomarker discovery, patient stratification and translational research.
Related topics
Analysis, Assays, Biomarkers, DNA, Drug Discovery, Drug Discovery Processes, Genomics, Metabolomics, Oncology, Translational Science
Related conditions
Cervical cancer, Human papillomavirus (HPV) infection
Related organisations
DNA Genotek Inc.