Romidepsin drug combo proves effective against resistant childhood cancer
Posted: 2 December 2025 | Drug Target Review | No comments yet
Australian researchers have identified a promising drug combination that can bypass treatment resistance in relapsed neuroblastoma, offering hope in the fight against one of the deadliest childhood cancers.

Scientists at the Garvan Institute of Medical Research in Sydney have discovered a new approach that may improve treatment options for children with high-risk neuroblastoma, one of the most aggressive childhood cancers. The discovery, identifies a drug combination capable of overcoming the cellular resistance that leads to relapse in children.
Neuroblastoma is the most common solid tumour found in children outside the brain. Typically diagnosed before the age of two, it develops from immature nerve cells in the adrenal glands or along the spinal cord. While outcomes for low-risk cases are excellent, around half of all patients present with high-risk disease, where tumours have already spread. In this group, 15 percent do not respond to initial treatment, and half of those who do respond later relapse. Typically, when relapse occurs, it is often fatal, claiming nine in ten children’s lives.
Understanding why treatment fails
The researchers team began by investigating how neuroblastoma becomes resistant to therapy. Using lab-grown cancer cells and tumour samples taken from the same children at diagnosis and after relapse, researchers tracked how the disease evolves under treatment pressure.
Finding a way to overcome the resistant state of relapsed high-risk neuroblastomas has been a major goal for my lab.
They discovered that many standard chemotherapy drugs rely on a single cellular ‘switch’ – known as the JNK pathway – to initiate cancer cell death. Once neuroblastoma returns, this switch frequently stops working, preventing chemotherapy from killing tumour cells effectively.
“Finding a way to overcome the resistant state of relapsed high-risk neuroblastomas has been a major goal for my lab,” says Associate Professor David Croucher, who led the research. “These tumours can be highly resistant to chemotherapy – and the statistics once patients get to that point are devastating for families. By finding drugs that don’t depend on the JNK pathway, we can still trigger cancer cell death even when this usual route is blocked.”
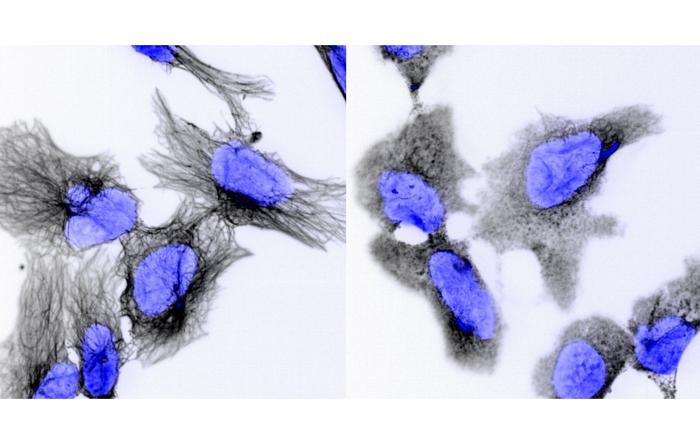

Neuroblastoma cancer cells before (left) and after (right) chemotherapy. The black threads are the cell’s internal scaffolding, which breaks apart during treatment. This damage should kill the cell, but resistant cancers can ignore signals to die – a discovery that led the research team to find alternative drugs that can bypass this resistance. Credits: Garvan Institute
A potent drug candidate identified
To find an alternative route to cell death, the team screened a large panel of FDA-approved drugs with established paediatric safety data. They identified romidepsin – currently used to treat certain lymphomas – as particularly effective against neuroblastoma cells, regardless of whether the JNK pathway was functioning.
To find an alternative route to cell death, the team screened a large panel of FDA-approved drugs with established paediatric safety data.
Working with the Children’s Cancer Institute, researchers then tested romidepsin in animal models of relapsed neuroblastoma. When combined with standard chemotherapy, romidepsin reduced tumour growth and extended survival compared with only chemotherapy. Notably, the addition of romidepsin allowed lower chemotherapy doses to achieve similar cancer-killing effects, raising the possibility of fewer toxic side effects for young patients.
Moving towards clinical trials
Although the results are highly encouraging, researchers emphasise that further work is needed before the treatment can be tested in children.
“This represents a big step forward, but the next challenge will be working on getting these findings into the clinic,” says Associate Professor Croucher. “We’re using this data as proof of principle to develop the best ways to deliver these treatments.”
Because romidepsin is already approved for use in other cancers and has paediatric safety data, they could progress to clinical trials quickly. However, extensive testing is required to confirm the safety and effectiveness of the combination in children with neuroblastoma.
Related topics
Animal Models, Cancer research, Chemotherapy, Disease Research, Drug Development, Drug Discovery, Drug Repurposing, Oncology
Related conditions
Neuroblastoma
Related organisations
Garvan Institute of Medical Research








