Scientists report using pluripotent stem cells to grow human oesophagal organoids
Posted: 25 September 2018 | Drug Target Review | No comments yet
Scientists have been able to grow human oesophagal tissue entirely from pluripotent stem cells (PSCs), which can form any tissue type in the body…
Scientists working to bioengineer the entire human gastrointestinal system in a laboratory now report using pluripotent stem cells to grow human oesophagal organoids.
The study is the latest advancement from researchers at the Cincinnati Children’s Center for Stem Cell and Organoid Medicine (CuSTOM). The centre is developing new ways to study birth defects and diseases that affect millions of people with gastrointestinal disorders, such as gastric reflux, cancer, etc. The work is leading to new personalised diagnostic methods and focused in part on developing regenerative tissue therapies to treat or cure GI disorders.
The newly published research is the first time scientists have been able to grow human oesophagal tissue entirely from pluripotent stem cells (PSCs), which can form any tissue type in the body, according to the authors. Cincinnati Children’s scientists and their multi-institutional collaborators already have used PSCs to bioengineer human intestine, stomach, colon and liver.
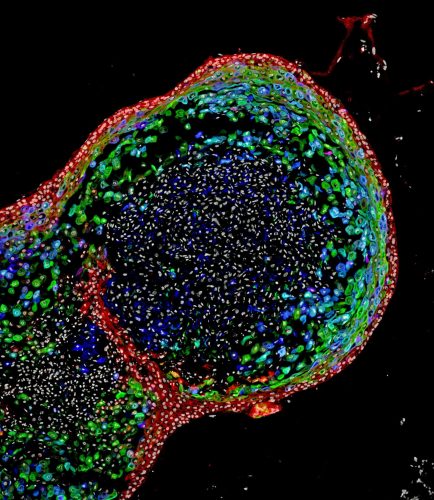
This confocal microscopic image shows a two-month-old human oesophagal organoid bioengineered by scientists from pluripotent stem cells. About 700 micrometres (0.027 inches) in size, the organoid is stained to visualize key structural proteins expressed in mature oesophagus, such as involucrin (green) and cornulin (blue). Researchers report in the journal Cell Stem Cell the organoids enhance the study of oesophagal disorders, personalised medicine and the development of regenerative tissue therapies for people. (Cincinnati Children’s)
“Disorders of the oesophagus and trachea are prevalent enough in people that organoid models of human oesophagus could be greatly beneficial,” said Jim Wells, PhD, the Chief Scientific Officer at CuSTOM and study lead investigator. “In addition to being a new model to study birth defects like oesophagal atresia, the organoids can be used to study diseases like eosinophilic esophagitis and Barrett’s metaplasia, or to bioengineer genetically matched oesophagal tissue for individual patients.”
The oesophagus is a muscular tube that actively passes food from the mouth to the stomach. The organ can be affected by congenital diseases, such as oesophagal atresia–a narrowing or malformation of the oesophagus caused by genetic mutations.
Additionally, there are several diseases that can afflict people later in life. Some include oesophagal cancer, gastroesophageal reflux disease (GERD), or a rare ailment called achalasia–a disease affecting the muscles of the lower oesophagus that prevents contraction of the organ and the passage of food.
All of the conditions need better treatments, researchers note. This requires a more precise understanding of the genetic and biochemical mechanisms behind their cause–a need filled by the ability to generate and study robust, functional, genetically matched models of human oesophagal tissue that can be grown from a person’s own cells.
The scientists based their new method for using human PSCs to general oesophagal organoids on precisely timed, step-by-step manipulations of genetic and biochemical signals that pattern and form embryonic endoderm and foregut tissues. They focused in part on the gene Sox2 and its associated protein–which are already known to trigger oesophagal conditions when their function is disrupted. –The scientists used mice, frogs and human tissue cultures to identify other genes and molecular pathways regulated by Sox2 during oesophagus formation.
The scientists report that during critical stages of embryonic development, the Sox2 gene blocks the programming and action of genetic pathways that direct cells to become respiratory instead of oesophagal. In particular, the Sox2 protein inhibits the signalling of a molecule called Wnt and promotes the formation and survival of oesophagal tissues.
In another test to help confirm the importance of Sox2 expression on the oesophagal formation, researchers studied the complete loss of Sox2 during the development process in mice. The absence of Sox2 resulted in oesophagal agenesis–a condition in which the oesophagus terminates in a pouch and does not connect to the stomach.
After successfully generating fully formed human oesophagal organoids–which grew to a length of about 300-800 micrometres in about two months–the bioengineered tissues were compared biochemically to oesophagal tissues from patient biopsies. Those tests showed the bioengineered and biopsies tissues were strikingly similar in composition, according to the authors.
The research team is continuing its studies into the bioengineering process for oesophagal organoids and identifying future projects to advance the technology’s eventual therapeutic potential, according to Dr Wells. This includes using the organoids to examine the progression of specific diseases and congenital defects affecting the oesophagus.
Published in the journal Cell Stem Cell.
Related topics
Disease Research, In Vitro, Induced Pluripotent Stem Cells (iPSCs)
Related people
Jim Wells