Antibiotics with novel mechanism of action discovered
Posted: 24 October 2019 | Rachael Harper (Drug Target Review) | No comments yet
A new family of synthetic antibiotics that possess broad anti-Gram-negative antimicrobial activity has been discovered.
Researchers have reported the discovery and characterisation of a new family of synthetic antibiotics that possess broad-spectrum anti-Gram-negative antimicrobial activity.
The research teams were headed by the University of Zurich (UZH) and Polyphor AG, both Switzerland.
“The new antibiotics interact with essential outer membrane proteins in Gram-negative bacteria,” said John Robinson from the UZH Department of Chemistry, who co-led the study. “According to our results, the antibiotics bind to complex fat-like substances called lipopolysaccharides and to BamA, an essential protein of the outer membrane of Gram-negative bacteria.”
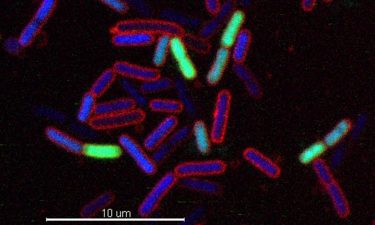
E. coli cells treated with a novel chimeric peptidomimetic antibiotic. Cells in blue are alive while green cells are already killed by the peptidomimetic. As the antibiotic destroys the integrity of the bacterial membranes, the scientists observed ‘explosive cell lysis’ (cells indicated by arrows), which leads in the release of DNA (diffuse green) (credit: Matthias Urfer, UZH).
BamA is the main component of the so-called ß-barrel folding complex (BAM), which is essential for outer membrane synthesis. After targeting this essential outer membrane protein, the antibiotics destroy the integrity of the bacterial membranes and the cells burst.
The outer membrane of Gram-negative bacteria protects the cells from toxic environmental factors, such as antibiotics. It is also responsible for the uptake and export of nutrients and signalling molecules. “Despite its critical importance, so far no clinical antibiotics target these key proteins required for outer membrane biogenesis,” Robinson continued.
The plan now is to progress one compound into human clinical trials. “POL7306, a first lead molecule of the novel antibiotics class, is now in pre-clinical development,” added Daniel Obrecht, chief scientific officer at Polyphor and co-head of the work.
Related topics
Antibiotics, Antimicrobials, Protein, Research & Development
Related organisations
Polyphor AG, University of Zurich (UZH)
Related people
Daniel Obrecht, John Robinson