Human protein important for cellular communication resembles bacterial toxin
Posted: 20 April 2018 | Drug Target Review | No comments yet
The structure of teneurins, proteins involved in embryonic development and the nervous system, is more like bacterial toxins than any other human protein.
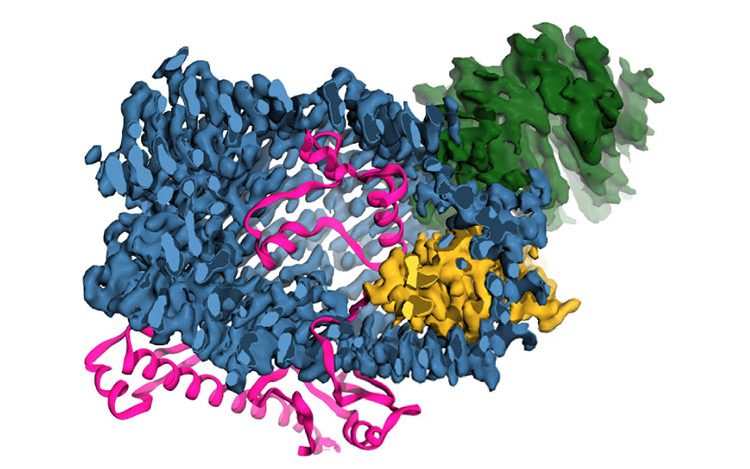
3-D model of a teneurin protein. Credit_ Araç, et al, UChicago
In a study published in Cell, scientists from the University of Chicago and Stanford University describe the three-dimensional structure of teneurins for the first time.
Instead of resembling other proteins that perform similar functions, however, the structure of teneurins most closely matches that of a bacterial toxin. This is the only known human protein with such a structure.
“There are many proteins on the cell surface that are important for adhesion, and they all have characteristic structures. But until now, we had no idea what these teneurins looked like,” said Demet Araç, PhD, assistant professor of biochemistry and molecular biology at UChicago and senior author of the study.
“No other eukaryotic protein was similar to this, so its resemblance to a bacterial toxin is unusual.”
Based on its genetic sequence, researchers had an inkling that teneurins resembled bacterial toxins, poison molecules bacteria use to attack and comprise host cells. Araç and Georgios Skiniotis from Stanford used high-resolution electron microscopy to define the structure of the human version of the teneurin protein. When they compared it to reference databases of other protein structures, it most closely resembled a bacterial toxin with a barrel shape and propeller-like protrusion.
This toxin may have been incorporated into multicellular organisms very early in evolutionary history. Choanoflagellates, one of the earliest single-celled organisms believed to evolve into multicellular eukaryotes, have a teneurin-like protein. It’s likely that bacteria transferred some of their genes to early choanoflagellates, which co-opted them for their own genomes. As these simple creatures evolved into more complex species, the original toxin-like protein has been conserved ever since.
Once the structure was resolved, Araç and her colleagues also wanted to understand how teneurins perform so many different functions during development and in the nervous system. They suspect it may have something to do with its similarity to the toxin – perhaps it borrows some of the same tools bacteria use to infect cells and employs them to attach to and communicate with other cells instead.
A genetic process called alternative splicing may also help teneurins perform their various tasks. Alternative splicing, or differential splicing, is a process that results in a single gene coding for multiple proteins. During this process, small segments of a gene called exons may be included or excluded in the final messenger RNA (mRNA) produced from that gene. These different mRNAs in turn translate into alternate versions of proteins that can perform various functions.
Araç and another collaborator from Stanford, Thomas Südhof, generated two different, alternatively spliced teneurins and tested their functionality. Just a tiny discrepancy – seven out of 2,500 amino acids – made a difference. One version of the teneurin lacking seven specific amino acids could adhere to a cell receptor that is important for signalling between cells. Another version of the teneurin that includes those seven amino acids couldn’t adhere to this receptor. Instead, it promoted formation of synapses between nerve cells.
“Usually there are different proteins that do different things in the nervous system. One does axon pathfinding, another one does synapse formation, another does brain development, etc,” Araç said. “This is one explanation for how teneurins do so many different things, by using different, alternatively-spliced isoforms.”
Now that the structure of teneurins is clear, Araç says she and her postdoctoral scholar, Jingxian Li, PhD, one of the lead authors of the study, want to continue working to understand how these proteins carry out their many different roles.
“It is still very surprising that we have these molecules from bacteria in our brain and nervous system, and yet we still don’t know much about what they are doing,” she said. “Humans are invaded by all these bugs. We are not just human.”
Related topics
Microscopy, Protein
Related organisations
Stanford University, University of Chicago
Related people
Demet Araç, Georgios Skiniotis, Thomas Südhof