Researchers programme solitary yeast cells to talk to one another
Posted: 1 July 2015 | Victoria White
A team researchers has engineered yeast cells (Saccharomyces cerevisiae) that can “talk” to one another, using a versatile plant hormone called auxin…
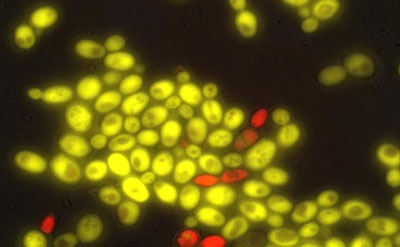

University of Washington researchers have produced cell-to-cell communication in baker's yeast, a first step in building multicellular organisms from scratch. The red yeast cells produce a plant hormone, which "tells" the green cells to express a gene differently. CREDIT: University of Washington
A team of University of Washington (UW) researchers has engineered yeast cells (Saccharomyces cerevisiae) that can “talk” to one another, using a versatile plant hormone called auxin.
The researchers have uncovered a novel cell-to-cell communication system that enables one yeast cell to regulate the expression of genes and influence the behaviour of an entirely separate yeast cell.
It’s a basic step in understanding the communication and cooperative processes that might lead to synthetic stem cells that could grow into artificial organs or organisms that require different types of cells to work together.
“Until you can actually build a multicellular organism that starts from a single cell, you don’t really understand it. And until we can do that, it’s going to be hard to do things like regrow a kidney for someone who needs it,” said Eric Klavins, a UW associate professor of electrical engineering and of bioengineering.
Yeast could be reprogrammed to recognise pathogens
It might also enable engineered yeast to perform complicated behaviours that coordinated multicellular systems such as our immune system can pull off, like recognising an invading pathogen and mounting a response. If so, one might programme those cells to collaboratively diagnose the flu or malaria: just add saliva to a packet of yeast and see if it changes colour.
For now, though, the team led by Arjun Khakhar, a UW doctoral student in bioengineering, and Nicholas J. Bolten, a UW doctoral student in electrical engineering, simply wanted to see if it could induce one yeast cell to send a signal that sets off a cascade of changes in another cell.
In the initial experiment, they used the plant hormone auxin – which yeast cells don’t normally recognise or respond to – to “turn off” a target gene in another cell. In this case, the gene that was switched off was an inserted jellyfish gene that turned the yeast fluorescent green.
To date, synthetic biologists have struggled to build modules that enable cell-to-cell communication in organisms that don’t naturally do it. The UW team overcame this hurdle by engineering a suite of novel transcription factors – proteins that control whether a specific gene inside a cell’s DNA is expressed or not – with varying sensitivities to auxin. That “tunability” offers important control in regulating cell behaviour.
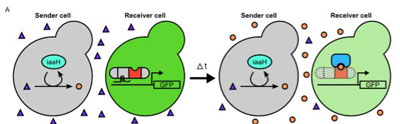

On the left, a transcription factor (gray and red) is integrated into the “receiver” yeast cell. This activates a gene that turns the receiver cell fluorescent green. On the right, a “sender” yeast cell produces a plant hormone called auxin (orange dots). In the receiver cell, auxin recruits another protein (blue) to disable the transcription factor and turn off the gene that turned the receiver cell green. CREDIT: University of Washington
The UW team figured out how to make a “sender” yeast cell produce auxin. Through trial and error, the team learned an enzyme borrowed from a soil bacterium can induce yeast to convert a commonly available chemical into auxin.
In the “receiver” yeast cells, the researchers inserted the new transcription factor and engineered it to activate the jellyfish gene that turned the cell green.
When the sender cell released auxin, additional proteins that the researchers introduced in the receiver cell were able to degrade the transcription factor and switch off the gene that turned the receiver cell green.
The researchers plan to test whether auxin can induce more complex behaviours in yeast
That type of simple communication forms the bedrock of multicellular organisms in which different types of cells collaborate to carry out complicated tasks. As a next step, the UW team plans to test whether auxin can induce more complex behaviours in yeast cells, such as forming patterns or cooperatively computing basic functions.
Since auxin is a plant hormone, mammalian cells also ignore it, making auxin a potentially useful tool in designing gene therapies or other applications without adverse reactions in humans. The UW method, which uses a “guide RNA” to target the gene of interest, could be adapted to produce a number of genetic or behaviour changes.
“If you ask someone in computer science what they can do with a programming language, they’ll laugh and say they can do anything with it,” Klavins said. “If we can figure out the programming language of life, we can do anything that life does – except in a more controllable, reliable way.”
The study is published in ACS Synthetic Biology.
Related topics
Bioengineering, CRISPR
Related conditions
Malaria
Related organisations
Washington University