FDA gives Roche 510k clearance for Treponema pallidum assay to aid syphilis diagnosis
Posted: 27 September 2016 | Niamh Louise Marriott, Digital Content Producer | No comments yet
The Treponema pallidum assay is intended as an aid in the diagnosis of syphilis infection. The Roche Syphilis treponemal antibody test offers several…

Roche has received 510k clearance from the US Food and Drug Administration (FDA) for a fully automated assay for the detection of antibodies to Treponema pallidium for use on all Roche immunoassay systems for low, mid and high volume testing environments, including the cobas e 411, cobas e 601, cobas e 602 and MODULAR ANALYTICS E170 analysers.
The Treponema pallidum assay is intended as an aid in the diagnosis of syphilis infection. The Roche Syphilis treponemal antibody test offers several advantages, including a specific screening test that provides an objective result, high throughput on an automated analyser, and high specificity.
“With the FDA approval of this fully automated assay, clinicians are now able to deliver clinically accurate, real-time results in as little as 18 minutes,” said Dr Wright CMO, Roche Diagnostics Corporation. “Having a tool like this available ensures they’re able to provide confident, appropriate and time-sensitive patient care in managing syphilis.”
Transmission and recommended screening of syphilis
Syphilis is mainly transmitted sexually, but can also be transmitted from mother to foetus during pregnancy and birth. Up to 80% of syphilis-infected pregnant women show adverse pregnancy outcomes resulting in over perinatal mortality rate of 40%. The World Health Organization (WHO) recommends all women be tested at their first antenatal visit and again the third trimester. If they are positive, WHO recommends that their partners be tested too.
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
The newly FDA-approved Trepnonema pallidum assay adds to the already available TORCH menu offerings of HSV-1 and 2, Cytomegalovirus, Toxoplasmosis, Rubella to screen and determine the immune status of the mother to prevent mother-to-child transmissions and to treat in time and prevent severe birth defects.
The CDC also recommends annual screening for all sexually active gay, bisexual, and other men who have sex with men (MSM). MSM who have multiple or anonymous partners should be screened more frequently for STDs (at 3- to 6-month intervals). Syphilis infection facilitates HIV infection.
About the disease
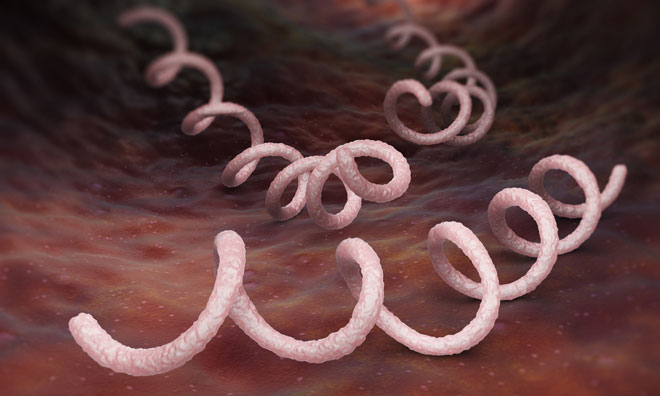
Syphilis is caused by the intracellular gram-negative spirochete bacterium Treponema pallidum subspecies pallidum. It can have very serious long-term complications if left untreated, but is simple to cure with the right treatment.
Congenital syphilis in the newborn may result in severe complications, including cataracts, seizures, deafness and even death. The clinical diagnosis of the disease can be difficult in the early stages of the infection as it is known as the great imitator because it has so many possible symptoms, many of which mirror the symptoms of other diseases. Symptoms of the disease in adults are divided into stages: primary, secondary, latent and late syphilis.
Related topics
Assays
Related conditions
Syphilis
Related organisations
Roche, U.S. Food and Drug Administration (FDA)