EUTCD-Grade lines: an innovative approach to creating and supplying clinical hESC lines
Posted: 14 September 2017 | Professor Glyn Stacey | No comments yet
The ever-increasing pressure to turn bench-top discoveries into new therapies has led to the re-purposing of embryonic stem cell lines, originally intended for basic research, for clinical applications…


However, within the European Union any donated human tissue intended for use in patient treatment must comply with European Tissues and Cells Directive (EUTCD) requirements to ensure quality and patient safety. Initiatives are being undertaken to ensure that human pluripotent stem cell lines intended for use in future clinical therapies also follow these directives.
In February 2017, the UK Stem Cell Bank (UKSCB) released the first of a panel of 37 EUTCD-Grade human embryonic stem cell (hESC) lines for use in developing novel advanced therapies to be brought to clinical trial. Derived and banked under a licence from the UK regulator, the Human Tissues Authority (HTA), it is hoped these lines will set a benchmark in standards for clinical stem cell lines. Here we explore what EUTCD-Grade means, and outline the processes behind producing and accessing the UKSCB’s panel of hESC lines suitable for the development of cellular therapies.
The UKSCB is a world-leading, not-for-profit, stem cell bank distributing pluripotent stem cell lines qualified for use in clinical trials. Funded by the Medical Research Council (MRC) and the Biotechnology and Biological Sciences Research Council (BBSRC), the bank was established in 2003 at the National Institute for Biological Standards and Control (NIBSC), a centre of the Medicines and Healthcare products Regulatory Agency (MHRA).
What are EUTCD-grade lines?
In 2006 the European Tissues and Cells Directive (EUTCD) was introduced to facilitate a safer and more streamlined exchange of tissues and cells between EU member states. This directive sets out the baseline standards which must be met when undertaking any operation involving human cells and tissues intended for patient treatment. This is implemented into UK law through the Human Tissue (Quality and Safety for Human Application) Regulations 2007.
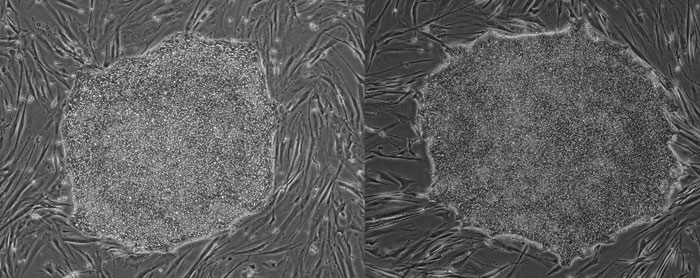

EUTCD-Grade hESC colony on human feeders
The UKSCB’s panel of EUTCD-Grade hESC lines has undergone a unique process, strictly regulated by the HTA, to ensure that each individual stem cell line meets the requirements of the EU Directive. As EUTCD-Grade cell lines are treated as clinical material from the start, this allows full traceability and reduces the probability of unknown microbiological contamination being passed onto patients during treatment. The current project has seen a coordinated effort from five partnered UK depositors. Derivation centres and the UKSCB are regularly audited by the HTA, which inspects all records associated with the EUTCD-Grade lines, including staff training records.
To make EUTCD-Grade embryonic stem cell lines, ethical consent for the use of donated material first needs to be obtained. Once consent is approved, derivation centres can begin to derive cell lines under the oversight of the UK Steering Committee for the Use of Stem Cell Lines. Cell lines are then deposited at the UKSCB and quarantined whilst due diligence is undertaken.
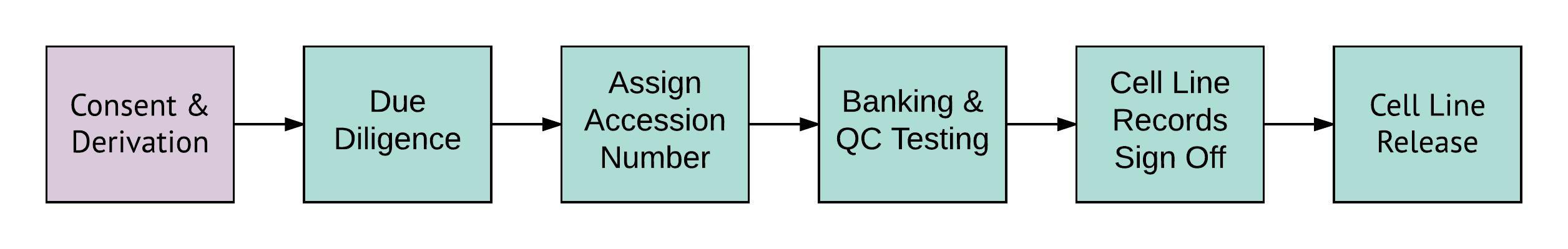

Figure 1: The process required to create a panel of EUTCD-grade cell lines. Green boxes represent activities undertaken by the UKSCB. Purple boxes represent those activities undertaken by partnered depositors.
Due diligence
To assure that the cell lines are fit for purpose and qualify as EUTCD-Grade, the history and documentation of each cell line are scrutinised by a qualified panel of experts at the UKSCB in a process called due diligence. The due diligence panel made up of quality control, banking, operations and UK regulation specialists, assesses whether the cell line meets all regulations and HTA requirements. It evaluates evidence of informed consent, traceability, regulatory compliance, microbiological safety, and appropriate functionality, looking specifically at the documentation for each individual cell line rather than the more general operation of the facility under HTA regulations.
Upon derivation, the new cell material is given a unique number to identify the line in the derivation centre. A separate number is given to the cell line during ethical review by the steering committee and a final accession number is assigned to it by the UKSCB following the due diligence procedure. This mechanism allows full traceability, a requirement of the EU Directives, but maintains anonymity.
Banking and quality control testing
The UKSCB has established top-class GMP-compliant clean room facilities, licensed for production of cells as starting materials for human application by the Human Tissue Authority (HTA) under the EU Tissues and Cell Directives. For each cell line, three banks are produced – a pre-master, master and distribution bank – making 50-100 vials containing around 5×105 to 1×106 cells. At every bank stage, quality control and informational testing is undertaken to monitor the quality and viability of the stem cell lines. This includes tests to assure their microbiological safety, and confirm cell viability, identity and differentiation potential.
All records and testing data associated with each EUTCD-Grade cell line is consolidated into a cell line master file (CLMF). This document provides a complete history of the cell line from donor to each UKSCB distribution cell bank, a key requirement of the EU directive. This is the final document to be reviewed and signed off before cell lines are released. It is hoped this comprehensive information and the traceability of the lines will support future applications for clinical trials, by verifying the clinical provenance of the EUTCD-Grade cell lines. The key benefit is that it provides a resource that a product developer can use to determine whether the cell meets the critical features for regulatory acceptance for use in clinical trials.
Cell line release
The UK Steering Committee oversees the UKSCB’s operation, including the procurement and distribution of hESC lines, to ensure the highest ethical standards are met. The committee approves requests to deposit or access hESC material, providing confidence to users and members of the public that the cell lines are ethically sourced and used for the appropriate purposes. Once an application has been approved, EUTCD-Grade lines can be provided by the UKSCB. The UKSCB can dispatch the desired cell line upon receipt of an approval letter and completion of additional documentation.
Author biography
Professor Glyn Stacey joined is the director of the UK Stem Cell Bank at NIBSC. His background is in microbiology and cancer research and he has worked on the development of cell substrates for the manufacture of biological medicines for over 20 years. Prof Stacey has chaired the UK National Clinical hESC Forum, and, and he is a visiting professor at the University of Bedfordshire in the UK.