Stem-cell technologies in immunotherapy
Posted: 17 June 2019 | Aparajita Dubey (Associate Scientific Manager at Biocon Biologics Limited) | No comments yet
This article outlines the exciting developments that are taking place with immunotherapies and demonstrates how stem-cell technologies are proliferating the paradigm shift in how we tackle cancer treatment.

THE AMALGAMATION of stem-cell technology with an immunotherapy approach has produced a new method that rejuvenates cancer-fighting immune cells and strengthens the ability of ageing cells to replicate in the body and mediate tumour regression. This represents a milestone for immunotherapy; developing more vigorous anti-cancer cells and using a similar approach to produce large quantities of cancer-fighting cells from patients’ own blood cells. Ageing cells have proven to be a major challenge in generating these cancer-fighting cells but induced pluripotent stem cell (iPSC) technology, which allows adult cells to be reprogrammed in the laboratory and returned to a stem cell state, is helping to overcome this.
In recent years, scientists have developed many therapeutic molecules – such as bifunctional multi-targeting antibodies, fusion proteins and oncolytic viruses – and different types of stem cells are being modified to facilitate their efficient delivery to tumours, illustrating the great potential of engineered therapeutic stem cells. This combination of immunotherapy and stem-cell technology has the potential to revolutionise cancer treatment regimes. Immunotherapy has changed our approach to cancer treatment by targeting the immune system rather than the tumour itself; this has been the consequence of better understanding the precise cellular interactions between cancer and the immune system. These undifferentiated stem cells are a powerful tool in clinical research to help unravel the mystery of cancer progression and development of stem-cell technologies is the ultimate goal.
Cancer immunotherapy
Immunotherapy represents an entirely new approach to cancer treatment; targeting the immune system rather than the tumour itself. This therapy makes use of the body’s own internal defenses to combat or check cancer growth and metastasis. Four common approaches to immunotherapy include the following:
- Immune modulators: these boost the patient’s entire immune system; not only those immune cells active within the tumour microenvironment
- Tumour targeted monoclonal antibodies (mAbs): these block the growth signal of tumour cells or induce apoptosis. They are designed to target specific antigens found on cancer cells
- Checkpoint inhibitors (CTLA4, PD1 and PDL1): these release the breaks on the body’s immune system and induce T-cells to actively combat tumour growth. This is directed against the cancer cells, which silence the body’s T-cell functioning by taking advantage of the immune system’s natural checkpoints designed to maintain equilibrium and prevent autoimmunity
- Adoptive T-cell therapy or adoptive cell transfer therapy: this is designed to enrich tumour infiltration lymphocytes by multiplying T-cells removed directly from the patient’s tumour microenvironment. These are analysed based on the receptors’ specificity or to determine which receptors are more specific to the tumour. These cells are then administered to the patient after being generated in greater numbers to initiate a massive antitumour immune response.
Upcoming advancements in immunotherapy include improvements in translational research, patient stratification, target validation and the development of predictive biomarkers.
Stem cells as a potential target
Characteristics such as a high proliferative capacity, low immunogenic reactivity and differentiating capabilities mark stem cells out as potential therapeutics – along with challenges such as unclear expression profiles in vivo and their potential immune-reactivity.
Major engineered stem cells for delivery of anti-cancer agents
Many adult stem cells, namely mesenchymal stem cells (MSCs) and neural stem cells (NSCs), have intrinsic tumour-tropic properties, which makes them a novel targeted cellular delivery vehicle for anti-cancer agents. Stem cells, along with their ability to home to the primary tumour mass, can efficiently track micro-metastatic lesions and can be engineered to express or secrete a range of anti-cancer agents. MSCs have been isolated from several organs including bone marrow, adipose tissue, foetal tissues, dental pulp, umbilical cord, Wharton’s jelly and other tissue types, among which bone marrow-derived stem cells play a major part in preclinical studies.
Recent advancements in CRISPR-mediated genome editing of primary cells such as T-cells and monocytes are taking us a step closer to engineering immune cells with the specificity necessary to develop the next generation of immunotherapies.
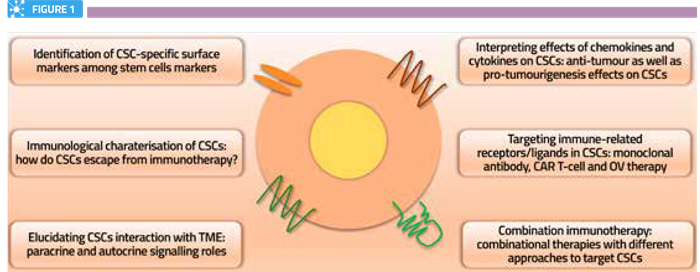

Prospective immunotherapeutic approaches to target CSCs.
Stem-cell mediated delivery
Different approaches used for stem-cell mediated delivery of biological anti-cancer agents include:
- Cancer stem cells (CSCs) or tumour-initiating cells (TICs): these are new targets for immune intervention for both solid and haematologic malignancies
- Stem-cell based therapeutic platforms: these include haematopoietic stem cells, reprogrammed pluripotent stem cells, stem cell-like memory T-cells and tumour-derived stem cells.
The identification of targets potentially associated with CSCs that are amenable to immunotherapy could play a major role in future discovery efforts and the development of therapies to cure and control different forms of cancer.
CSCs have drug resistance to most chemotherapeutic agents and are also responsible for tumour cell heterogeneity. In order to achieve complete regression of tumours, CSCs must be targeted. Recent advances in immunotherapies are also applicable for targeting CSCs. Immune markers expressed by CSCs exhibit specific immune characteristics in various cancers, which can be used in immunotherapies to target CSCs in the tumour microenvironment. The following strategies have been recently applied to target CSCs: adaptive T-cells, dendritic cell (DC)-based vaccines, oncolytic viruses, immune checkpoint inhibitors and combination therapies.
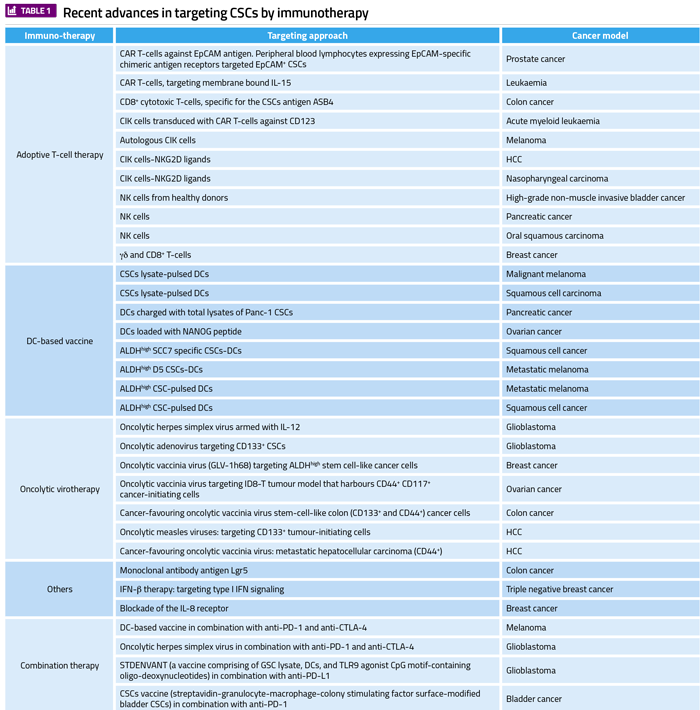

Abbreviations: ALDH, aldehyde dehydrogenase; CAR, chimeric antigen receptor; CCR7, CC-chemokine receptor 7; CIK cells, cytokine-induced killer cells; CRC, colorectal cancer; CSCs, cancer stem cells; CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; CXCR1, C-X-C chemokine receptor 1; DC, dentritic cells; HCC, hepatocellular carcinoma; IFN-β, interferon-beta; IFN-γ, interferon-gamma; IL-8, interleukin 8; IL-12, interleukin 12; NK, natural killer; NKG2D, natural killer group 2D; PD-1, programmed cell death-1; PD-L1, programmed death-ligand 1; TLR9, toll-like receptor 9.
Immunotherapy targeting CSCs
Immunotherapy targets for CSCs include immune cells such as cytokine-induced killer (CIK) cells, natural killer (NK) cells, T-cells, and CD8+ T-cells. DC-based vaccines also target CSCs; oncolytic virotherapy (OVT) induces antitumour immunity through immunogenic cell death and activation of the T-cell. Combinations of immunotherapies are also used to target CSCs. Recently, strategies in combination therapy include DC-based vaccines, oncolytic viruses and immune checkpoint blockades.
Adoptive T-cell therapy
This is a type of personalised cancer therapy that uses cancer-bearing host immune cells with direct anticancer activity. Engineered T-cells with chimeric antigen T-cell receptors (CAR T-cells) against the antigens of CSCs have been recently developed and evaluated in various cancer models.
Combination immunotherapy
Combined immunotherapy approaches have recently been developed that aim to completely eradicate CSCs; an example of which is a DC-based vaccine in combination with anti-PD-L1 and anti-CTLA-4.
Significant features of the CSC model
Features of the model include: low abundancy, self-renewal, high tumourigenicity, ability to reconstitute a heterogeneous population of cells, expression of multi-drug resistance proteins and upregulation of DNA repair proteins. Extensive clinical evidence establishes the importance of CSCs in cancer progression, relapse and metastasis, which suggests that targeted therapies could be a potential approach to achieve a comprehensive treatment regimen.
Induced pluripotent stem cells and immunotherapy
iPSCs specific to patient iPSCs could be potentially beneficial for immunotherapy approaches. Functionally-active T-cells can be induced to differentiate from pre-rearranged TCR genes retained in T lymphocyte-derived human iPSCs. Reprogramming of selected T-cells into iPSCs in vitro can lead to the production of tumour antigen-specific T lymphocytes. These iPSCs then differentiate back into T lymphocytes for infusion into patients after checking the safety-related aspects.
Targeting CSCs
Normal stem cells can also be used to target CSCs in cancer therapy. Normal stem cell and CSC interaction suppresses tumour proliferation, angiogenesis and metastasis, and reduces inflammation and apoptosis. The potential of NSCs and haematopoietic stem cells (HSCs) in anti-glioblastoma therapy was assessed and it was concluded that HSCs can be ideal for developing technologies aimed at controlling glioblastoma CSC activity. HSCs are unique since they are less prone to neoplastic transformation in neural tumours than neural stem/progenitor cells and can be engineered to facilitate the generation of cell systems that can trigger targeted CSC apoptosis.
Cancer stem cell limitations and advantages
Since CSCs are resistant to conventional approaches, this can lead to failure of colorectal cancer (CRC) therapy for patients. However, dendritic cells (DCs) could effectively supplement CRC therapy. Some major features of CSCs include metastasis, recurrence, relapse and resistance to conventional chemotherapy, which only destroys proliferating and mature cancer cells while quiescent CSCs survive. Clarification related to the maintenance of CSCs is important to understand cancer cell persistence and relapses. CSCs can be used as a potential targeting mechanism and also as a potential therapeutic strategy to eradicate cancer. Applying knowledge regarding CSCs could play a key role in future drug development; to address cancer resistance, relapse and progression. It’s comparatively refractory to current therapies such as chemotherapies, radiotherapy and small molecule targeted therapies because CSCs can persist for longer durations in the body without being subjected to immune surveillance or other homeostatic mechanisms.
Conclusion
Stem-cell technologies along with immunotherapy have the potential to provide breakthroughs in cancer therapy. The ability of stem cells to migrate to solid tumours and micrometastatic lesions, facilitating site-specific anti-tumour agent delivery and overcoming the short half-lives of conventional chemotherapeutic agents, is dynamic. A more widespread clinical utilisation of stem cell-based therapies are expected in future. Adding to this approach, researchers are testing a stem cell-derived NK cell immunotherapy in people with incurable cancer. Cells derived from iPSCs are being used for the first time in this “off-the-shelf” NK immunotherapy clinical trial. This regime to rejuvenate cancer-fighting immune cells by strengthening ageing cells and developing large amount of cancer-fighting cells via immunotherapy is a great advancement in current and future cancer research.
Biography


Related topics
Immunotherapy, Stem Cells
Related organisations
Biocon Research Limited
Related people
Aparajita Dubey








