Recent trends in upstream bioprocessing and major challenges in process development
Posted: 17 September 2019 | Aparajita Dubey (Associate Scientific Manager at Biocon Biologics Limited) | No comments yet
Aparajita Dubey summarises the recent trends in upstream bioprocessing and highlights the challenges and solutions involved in its process development.


A ROBUST high-yielding upstream process is a combination of powerful technology, high-quality starting material, and a brilliant process design. Bioprocess engineering involves understanding the conditions most favoured for optimal production and duplicating these conditions during scaled-up production. Safety, purity, potency, efficacy and consistency are the key features involved in bioprocess development of recombinant monoclonal antibodies (mAbs) and other novel drugs. The most challenging characteristic of upstream processes is the use of living organisms, which do not always behave in an expected way.
Tremendous progress has been made in upstream processing in the last decade, which has resulted in the development of modern fed-batch processes capable of delivering 10g/L of mAb or more. Better cell culture media, more advanced feeding strategies, more robust cell lines and bioreactor control tailored for specific processes are the major pillars of upstream development that have resulted in the overall progress made in this domain.
Quality by design (QbD) and process analytical technology (PAT) have contributed to a paradigm shift in the way companies approach cell culture process development. Risk management helps in prediction processes, which prevents trouble before it arises. The future of bioprocessing lies in complete automation and the development of next-generation techniques, with continuous bioprocessing focusing on high productivity, high intensity and manufacturing operations working as an integrated process.
Steps, challenges and solutions involved in process development
The basic or initial steps in bioprocess development consist of cell-line optimisation, clone selection and screening for media, feed components and strategies, as well as other process conditions. Shake flask studies are a common tool in cell culture, which enable control of temperature, ambient gas mix and agitation rate; but there are some limitations to its capabilities related to standard upstream bioprocess monitoring and controlling of critical process parameters such as pH, dissolved oxygen (DO) and feed schedules.
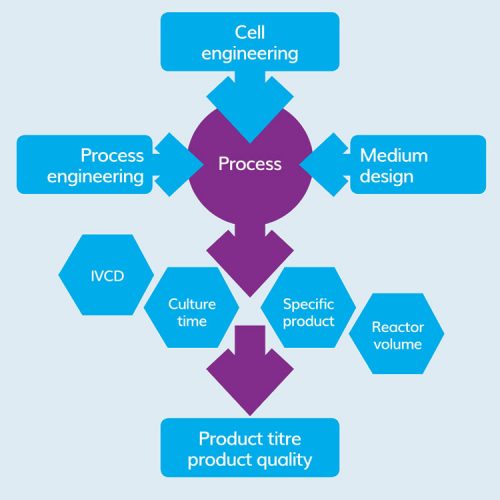

Figure 1: Bioprocess development streams.
For most biomanufacturing cell lines, only glucose and glutamine are catabolised in larger quantities, with lactate and ammonia being the two major by-products. Glucose serves as the main carbon source and glutamine is the main source of nitrogen. Thus, selecting suboptimal clones during early development when using shake flasks becomes challenging because these uncontrolled conditions diminish cell productivity and product quality, which then persist throughout development and beyond. Hence, during selection of high producer clones, shake flasks limit analysis of the most important factors such as media influences, feed and supplementation strategies, that are instrumental in improving volumetric productivity.
Bioprocess development platforms require consistency throughout the development phases, starting from early-stage process development. It should mimic the physical and mechanical characteristics of production-scale reactors as much as possible. Bench-top bioreactors address the issue of process consistency and harmonise unit operations between development and production. Traditionally, bioreactors routinely monitor pH, temperature and DO online, but have limitations in providing significant insight into process mechanisms. This lack of routine in-line measurements of critical process parameters (CPPs) reduces process understanding and the ability to control the critical quality attributes (CQAs) of the product by manipulation of the appropriate CPPs.
Some important attributes of the upstream process
Impact on metabolic circuitry
Cells are the most complex and unpredictable raw materials used in the upstream process, owing to their intricate metabolic circuitry. Linking process control parameters to metabolic behaviour is therefore essential to progress in the field of process design. The focus lies on metabolic response-based optimisation of two important parameters:
- Nutrient supply
- The cell’s external environment.
Amino acids form one of the most essential nutrient groups, supplying nitrogen blocks for protein building and energy for cellular activity. The metabolic rewiring capacity of CHO cells in response to their external environment is immense. One of the defining factors that impacts cell performance is the pH of the cell’s external environment and various strategies are used to control pH – with buffer type and strength being two of the determining factors – and they can be used to influence lactate synthesis and hence overall cell health.
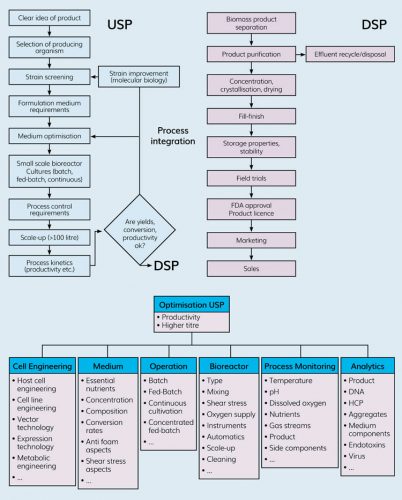

Figure 2: Optimisation areas and parameters in upstream processing.
Stable cell lines – a major challenge for upstream development
Establishing the stability of those cell lines being used for antibody production forms an important part of upstream development. Stability conventionally indicates the antibody- production capability of cells. This is tested by periodically checking their antibody-production capability as the cells age (cell age is measured in terms of generation number). This provides a limit within which cells can be used based on how long they remain productive. Cells lose more than just their antibody production capability with ageing. Ageing can generally be termed as a system’s deterioration with time; or as the progressive loss of physiological functions performed by cells. CHO cells, which are used as factories to produce mAbs, change with ageing over prolonged subculture and process conditions. The causes and effects of ageing on cells can be varied: the effects may be a reduction in cell growth, productivity or cell health and metabolism; the causes could be environmental stress to cells such as additives, waste accumulation, effects of free radicals, genetics or wear and tear. Product quality attributes are vital to the manufacture of mAbs as these determine the efficacy of the drug. The deteriorating health of cells also affects the product that they produce and hampers their quality, resulting in change in cell physiology, which eventually leads to antibody fragmentation.
Role of upstream parameters in modulating protein glycosylation
Glycosylation is recognised as an important quality attribute that impacts the efficacy of many mAbs. Glycoproteins are produced within the cell; it is important to understand and control these proteins at the molecular stage during its production in the stationary phase.
Product quality attributes are vital to the manufacture of mAbs as these determine the efficacy of the drug.”
Environmental factors and cell state glycosylation
- Environmental factors include process parameters such as pH, dissolved oxygen, temperature, mode of culture (batch, fed-batch, perfusion, etc) and media components
- Cell state can also affect glycosylation by up-regulating and/or down-regulating the glycosylation machinery.
Understanding the impact of single or combined process parameters during process development helps to understand the effects of such changes on product glycosylation. The role of cell state, media components and process parameters on protein glycosylation is tremendous. The cell state is also influenced by the change in process parameters specified above, which has an impact on protein glycosylation. Among various media components, specific substrate and cofactor modifications truly impact and modulate the glycosylation pathway.
Future developments in upstream bioprocessing
Next generation upstream process development
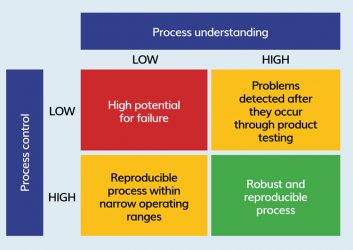

Figure 3: QbD philosophy for process development.
The increased acceptance and approvals of biosimilars in the global biologic market has increased the pressure and choice to make cost-effective medicine and to deliver affordable treatment options for advanced therapies. Although fed-batch processes have been successful for several decades due to improved yields coming from high-producing cell lines, expression systems, feed and media optimisation, etc, this manufacturing mode has its own limitations. Companies must adapt to newer manufacturing technologies, such as continuous perfusion, to increase the product output from their existing facilities to meet market demand.
Companies must adapt to newer manufacturing technologies, such as continuous perfusion, to increase the product output from their existing facilities to meet market demand.”
Continuous bioprocessing technology for production of mAbs and other novel fusion proteins is referred to as the Next Generation Platform Process (NGPP). Continuous bioprocessing in upstream also involves switching an existing process from fed-batch to perfusion culture. Perfusion mode continually balances cell growth and cell productivity, which makes it slightly more challenging to obtain the desired glycosylation pattern. Cells in perfusion mode are healthier, since they are continually re-fed, with the cells being shifted toward protein production rather than cell growth mode. For instance, a fed-batch process that is operating at 20 million cells can be turned into a perfusion process operating at 80–100 million cells.
The proof-of-concept of NGPP was established using the shake flask model, followed by scaling up the process to lab and pilot scale bioreactors using hollow fibre membranes as the cell retention device. Although operationally intensive, the Tangential Flow Filtration (TFF) and Alternating Tangential Flow (ATF) perfusion systems have enabled easy adoption of this platform process. With NGPP, a 10-fold higher titre was obtained with the same cell lines compared to the fed-batch process, without any significant differences observed in product quality attributes. This change of direction in process development is expected not only to drive business, but also lead to more affordable innovation.
Quality by design
Two important tools to optimise documentation and develop control and automation strategies are process characterisation and modelling. Models also form a core part of the QbD framework.
The construction of a process model can be divided into the following basic steps:
- Defining system variables
- Performing a kinetic analysis n Setting up mass balances
- Validating the model.
The regulatory authorities’ current shift in philosophy towards QbD has increased the speed, efficacy and information content associated with the development and scale-up of biopharmaceutical processes in recent years.
Process Analytical Technology
The US Food and Drug Administration’s (FDA’s) PAT initiative for upstream bioprocessing promotes improved process understanding by defining CPPs and monitoring them either in-line or on-line. PAT can be used to achieve the fundamental goal of producing high-quality mAbs and recombinant proteins. Processes can become more efficient, with reduced over-processing, greater product consistency and more productivity with a PAT programme that is simple, data rich and low risk. These capabilities are being leveraged to study continuous bioreactor cell culture production and compatible PAT tools for upstream bioprocessing to support regulatory decision making.
Upstream optimisation
Upstream optimisation is needed to overcome common dangers such as accumulation of ammonium or lactate, as well as various biostability problems that can inhibit cell and protein growth. Many biologic developers and contract manufacturing organisations (CMOs) are working to streamline their bioreactor production lines. The emergence of single-use disposable bioreactors has been a huge success due to the reduced capital investment and increased operational flexibility. Similar growth environments are required for a wide range of biologic products. A generic harvest system with processes such as centrifugation and depth filtration to remove impurities and achieve higher concentration of the end product can provide a great boost to upstream bioprocessing. Development of more efficient, productive process strategies throughout the production line is compulsory, along with optimisation of growth media and bioreactors. A major focus is to design individualised processes that are tailored around the specific needs of a given bioprocess, to remove by-products and add nutrients to the growth media at certain stages.
Conclusion
The evolving biopharmaceutical industry is focusing on new and improved technologies to reduce costs, increase efficiencies and improve weak development pipelines. Upstream bioprocessing is playing a major role through the adoption of single-use systems for commercial scale production. Efficiency in bioprocessing is boosted as titres and yields continue to increase. This is aided by the use of continuous processing (including for downstream processing), automation, monitoring and process control, including the use of bioprocess modelling, data mining, PAT and QbD approaches.
About the author
Aparajita Dubey is an Associate Scientific Manager at Biocon Biologics Limited, Bangalore, India. Aparajita has more than 11 years’ experience in pharmaceutical research, mainly in cancer biology and immunotherapy within Biocon, Panacea and Jawaharlal Nehru University. As a biologist she has broad experience in the field of cell-line development, cancer immunotherapy and molecular biology along with medical writing and consulting experience at Prescient Healthcare. She has an MSc in Life Sciences and a PG Diploma in IP from National Law School University, Bangalore, India.
Related topics
Analysis, Biosimilar, Cell Cultures, Cell Line Development, Upstream Bioprocessing
Related organisations
Biocon Biologics Limited, NanoTemper