Gene therapy reduces hepatocellular carcinoma in mice
Posted: 8 December 2023 | Drug Target Review | No comments yet
Increasing microRNA-22 overexpression in a gene therapy approach treated HCC in mice, offering promise for its prevention and treatment.
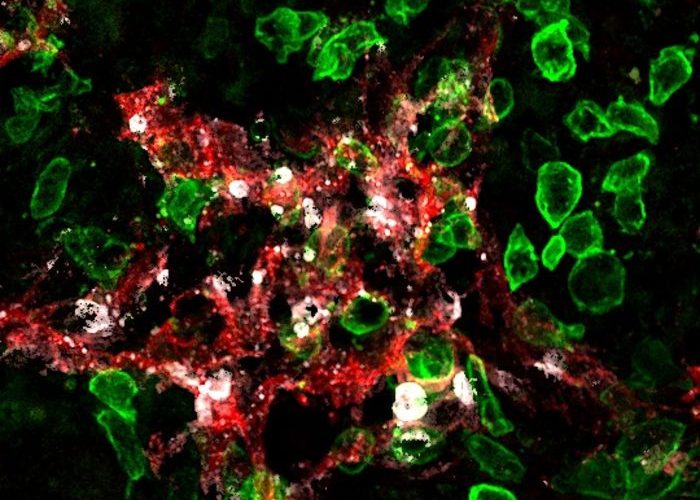

Killer-T cells (green) attack lymphatic vessels (red) in tumours and induce their death (cell death marker in white) [Credit: UNIGE - Robert Pick / Stéphanie Hugues].
Scientists at UC Davis Comprehensive Cancer Center have used gene therapy to demonstrate that inhibiting a specific protein can reduce hepatocellular carcinoma (HCC) in mice. HCC is a cancer that begins in the liver and is one of the world’s most common cancers. Incident rates have more than tripled since the 1980’s and in its advanced stages, has a five-year survival rate of less than 20 percent.
The researchers also found that silencing the galectin 1 (Gal1) protein, which is frequently over-expressed in HCC, improved the anticancer immune response, and increased the number of killer T cells inside tumours.
Senior author of the study and professor in the Department of Pathology and Laboratory Medicine Dr Yu-Jui Yvonne Wan said: “We’ve long known that Gal1 is a biomarker for hepatocellular carcinoma…Gal1 expression in normal tissue is quite low and increases with fatty liver disease, inflammation, and liver carcinogenesis. Now, we can see that Gal1 is more than a biomarker — it’s a potential therapeutic target.”
The Wan lab has studied liver diseases for decades studying liver diseases and this study builds on previous research.
Overexpression of Gal1
Gal1 suppresses the immune system from attacking healthy tissue but when it is overly expressed in HCC, it promotes cancer growth and prevents the immune system from attacking the tumour. In the new study, the team found aggressive disease progression and poor survival were associated with high Gal1 levels. This link between increased Gal1 and poor outcomes has also been observed in HCC patients.
The Wan team used gene therapy to increase microRNA-22 (miR-22), a non-coding RNA that regulates gene expression, to investigate its effect on liver cancer. Led by Dr Ying Hu, an assistant professional researcher in Wan’s lab, this approach demonstrated miR-22 overexpression reduced liver inflammation, treated HCC, and produced better survival outcomes than Lenvatinib, a Food and Drug Administration (FDA)-approved HCC drug, in an animal model.
The scientists noted that miR-22 decreased activity for several genes, including Gal1. They then investigated whether decreasing Gal1 could be an important mechanism for how miR-22 treats HCC.
A short interfering RNA (siRNA), called Igals1, was used to inhibit Gal1. The siRNA was packaged for delivery into adeno-associated virus 9, which prefers to land in the liver. Igals1 effectively silenced Gal1 in both the cancer and stroma, the supporting tissues in and around the tumour.
Silencing Gal1
Wan said: “Silencing Gal1 reduced HCC tumours, which are extremely hard to treat…In addition, we found the therapy reduced Gal1 in the stroma at the tumour margin, so it has a big impact on the tumour microenvironment.”
The FDA have approved some adeno-associated virus-delivered gene therapies to treat genetic diseases like spinal muscular atrophy and Haemophilia B, although few researchers have explored using this approach against tumours.
Inhibiting Gal1 may be found to benefit human HCC patients, but this approach could present other opportunities, as Gal1 is overexpressed in many types of cancer, including breast, colon, and lung. It starts to build up in diseased livers long before HCC develops, so Gal1 inhibition could be considered for HCC prevention.
The authors believe this research and other work shows the potential of gene therapy for cancer treatments. “Silencing galectin 1 is potentially a groundbreaking strategy in the fight against liver cancer,” said Dr Tahereh Setayesh, first author of the study and a former post-doctoral researcher in Wan’s lab.
Setayesh is currently at the Cincinnati Children’s Hospital Medical Center. She continued: “It offers the potential to treat HCC, as well as holding the promise of prevention, providing a path towards transformative therapies.”
The study was published in Acta Pharmaceutica Sinica B.
Related topics
Biomarkers, Cancer research, Gene Therapy
Related conditions
Cancer Research, hepatocellular carcinoma (HCC)
Related organisations
UC Davis Comprehensive Cancer Center