App Note: Multi-analyte, Automated, Microfluidic Immunoassay Platform
Posted: 16 October 2015 | ProteinSimple
Simple Plex assays run on Ella™ enabled simultaneous quantitation of four analytes from eight samples in a disposable cartridge in under an hour…
Protein analytes or biomarkers have traditionally been measured individually in ELISAs, which can attain a high degree of analytical specificity by testing only a single analyte with a dedicated antibody pair.
However, the clinical field is becoming increasingly aware that multiple markers are associated with complex, multimodal, multivariate diseases including sepsis, rheumatoid arthritis, cancer, neurodegenerative diseases and traumatic brain injury. Unfortunately, adoption of multi-analyte biomarker tests in clinical research has been severely limited for many reasons, including technical concerns regarding assay reproducibility, cross-reactivity between the disparate antibody assay components, decreased sensitivity or increased variability at low concentrations, possibly resulting from heterophilic antibodies, the time and labor-intensive nature of assay panel development, and reported non-correlation with conventional ELISA data.
To address this issue, ProteinSimple developed Simple Plex™, a novel automated immunoassay platform that enabled simultaneous multi-analyte quantification, while retaining the sensitivity and specificity of single-analyte ELISAs without the associated negatives of multi-analyte analysis of high sample volume requirements, slow reaction rates, high labor requirements, and cost inefficiencies when analyzing multiple analytes. Simple Plex assays run on Ella™ enabled simultaneous quantitation of four analytes, in discrete channels, from eight individual samples in a single disposable microfluidic cartridge in under an hour. This eliminated potential negative interactions or interference from the antibody pairs for other assays, while simultaneously providing the benefits of a multiplexed antigen analysis and rapid microfluidic reaction kinetics.
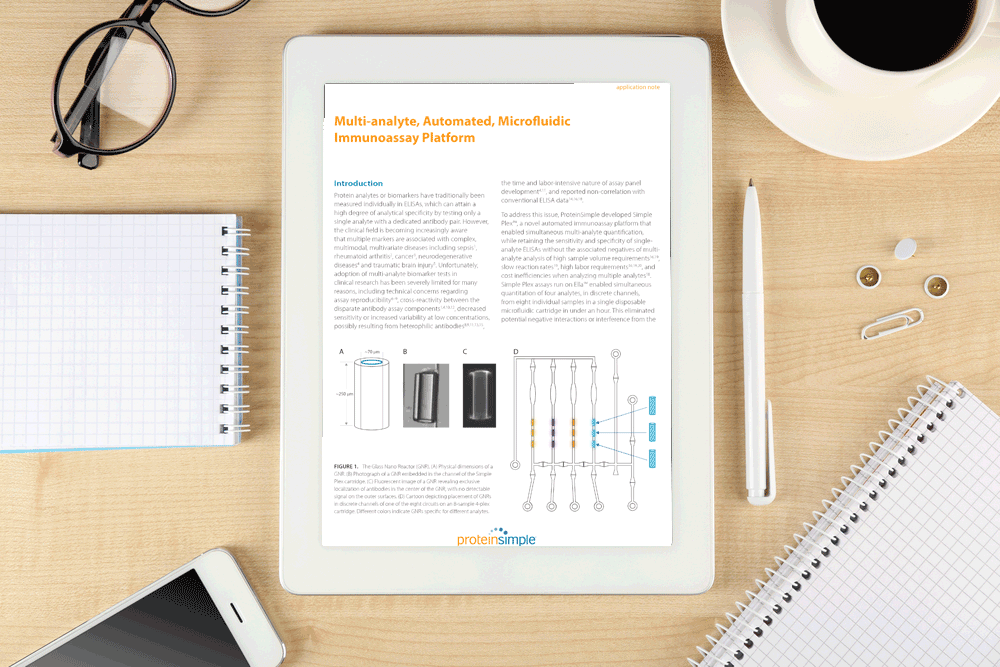
Glass nano reactors (GNR or GNRs) were developed as a solid phase support for the capture antibodies, and were shown to possess enabling physical, optical and chemical properties. GNRs were functionalized to allow antibody immobilization on the GNRs internal, but not external, surfaces. The composition of the GNR provided a highly uniform surface, supporting multiple well-characterized, stable immobilisation chemistries, and exhibited low intrinsic fluorescence.
This application note is restricted - login or subscribe free to access


Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- quarterly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related content from this organisation
Related topics
Biomarkers, Immunoassays, Microfluidic Technology
Related organisations
ProteinSimple