Inflammation ‘brake’ gene may impact kidney disease outcomes
Posted: 24 April 2023 | Izzy Wood (Drug Target Review) | No comments yet
Australian researchers uncover a gene that controls inflammation in kidney disease, which could pave the way for more precise disease diagnostics and personalised treatments.

Researchers at the Garvan Institute of Medical Research, University of New South Wales and Westmead Hospital, Australia, have made a significant discovery about gene variants that regulate inflammation, bringing them a step closer to personalised treatment for kidney disease and kidney failure.
The team, led by Professor Shane Grey, Head of the Transplant Immunology Lab at Garvan, focused on the TNFAIP3 gene, which produces a protein called A20 that acts as a ‘brake’ on inflammation. Common variants of TNFAIP3 have previously been linked to autoimmune diseases, but their role in kidney disease was unknown until now.
The researchers found that some genetic variants of TNFAIP3 can paradoxically protect the kidneys from damage in the short term, despite increasing inflammation. The team discovered that these variants altered the outcome of kidney injury by affecting inflammation and cell survival. The findings were published in the journal Kidney International.
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
Acute kidney injury, which is characterised by a sudden and rapid decline in kidney function and is partly caused by inflammation, is a significant risk factor for the progression to chronic kidney disease. Approximately one in 10 Australians are affected by chronic kidney disease, and there are currently limited treatment options for acute kidney injury, with imprecise tools to predict who is most at risk of poor recovery or kidney failure.
The team first investigated how different TNFAIP3 variants influenced the function of A20, and identified a series of rare variants that reduced its anti-inflammatory effect. They then tested the effects of one of the variants that promotes inflammation during kidney injury in a mouse model.
“Despite increasing inflammation, this rare variant surprisingly protected the kidneys from injury. We found this protection to be due to another of A20’s functions: preventing cells from self-destructing,” explained Professor Natasha Rogers, nephrologist and Head of Transplantation at Westmead Hospital, who co-led the study.
The findings of this study could lead to the development of a simple genetic test that allows doctors to determine whether an individual carries a ‘hot’ version of the inflammation control gene, providing families with greater certainty about their risk factors for kidney disease. This could pave the way for personalised treatment approaches and precision diagnostics for acute kidney injury.
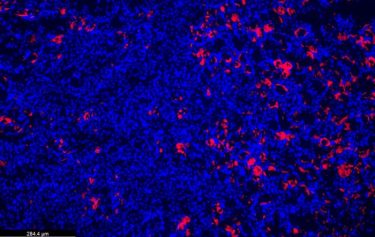

Paradoxically, a gene variant that increases inflammation also has a protective effect on the kidneys. Seen here, kidney cell nuclei (blue) and an influx of immune regulatory cells (pink) that prevent damage in an injured kidney.
(Credit: Garvin)
“More work is needed, but these findings bring us closer to being able to predict who is at risk of poor kidney recovery and open personalised treatment approaches,” explained Grey.
The potential impact of this research is significant, as it could revolutionise the approach to monitoring and treating kidney disease. Rather than a one-size-fits-all approach, doctors may be able to determine the best course of action for a patient based on their specific variant of TNFAIP3, and personalise interventions to boost their kidney recovery and long-term health.
“By gaining a better understanding of how variants of the TNFAIP3 gene influence kidney health, this research brings us closer to precision diagnostics and tailored treatments for acute kidney injury,” added Grey.
Further research is needed to fully understand the mechanisms underlying the protective effects of these gene variants and to develop effective personalised treatment strategies for patients at risk of kidney disease and kidney failure. However, this discovery represents a significant step forward in the field of personalised medicine and holds promise for improving the outcomes of patients with kidney disease.
Related topics
Animal Models, Gene Therapy, Genetic Analysis, Immunology, Personalised Medicine, Targets
Related conditions
Kidney disease, kidney failure
Related organisations
Garvan Institute of Medical Research, University of New South Wales, Westmead Hospital
Related people
Professor Natasha Rogers, Professor Shane Grey