A spatial approach to understanding drug dynamics using mass spectrometry imaging
Posted: 16 June 2025 | Stefan Foser - (Vice President - Bruker Daltonics), Steve Castellino (Chief Analytics Officer & Principal Investigator - GlycoPath) | No comments yet
What if you could actually see where a drug travels in the body down to the cellular level. Find out how mass spectrometry imaging (MSI) is making that possible – reshaping drug development from the inside out.


Mass spectrometry imaging (MSI) enables the direct detection and quantitation of active pharmaceutical ingredients (APIs) and metabolites within tissue sections, making it widely regarded as a promising technique in the field of pharmacology and toxicology. Unlike traditional pharmacokinetic (PK) studies that rely on plasma measurements alone, MSI, when combined with traditional histology, enables spatial mapping of drug distribution, metabolism and target engagement. This unique combination offers valuable insight into therapeutic efficacy and toxicity.
Toxicological investigations play a key role in assessing drug safety and, importantly, inform development decisions. They aim to establish mechanistic hypotheses of toxicity, guiding go/no-go decisions that are crucial for balancing therapeutic benefits against potential risks. Decision makers rely on a broad body of evidence, including histopathology, clinical chemistry, pharmacodynamics (PD), PK, drug metabolism and in vitro experiments. In some cases, a black box warning must be used for a practitioner to weigh against therapeutic benefits, rather than halting a drug’s development if the risks prove insurmountable.
Imaging technologies such as MSI and matrix-assisted laser desorption/ionisation (MALDI) mass spectrometry (MS) integrate seamlessly with histological techniques like haematoxylin and eosin (H&E) staining and immunohistochemistry (IHC). Together, they provide a unique picture that combines analytical and spatial information to give a deeper understanding of drug localisation, help to optimise dosing strategies and mitigate off-target effects. Additionally, MSI aids in the identification of reliable biomarkers for early toxicity detection, supporting clinical safety and risk mitigation.1
Continuous advancements in sensitivity and spatial resolution support the role of MSI in pharmaceutical research. By complementing traditional analytical methods, it provides a more accurate representation of drug behaviour to support translational and pre-clinical studies. This article explores the principles, applications and future potential of MSI in drug development.
Tissue-specific drug distribution analysis
MSI is a powerful spatial molecular imaging technique that directly detects APIs and their metabolites without the need for labels or surrogate markers. As a multiplexed tool, it offers insights into drug distribution within tissues, identifying parent compounds, known and potential metabolites, and endogenous biomarkers in a single experiment. A typical MSI workflow – comprising sample preparation, MSI acquisition and data integration – is illustrated in Figure 1.
MSI is a powerful spatial molecular imaging technique that directly detects APIs and their metabolites without the need for labels or surrogate markers.
This capability is fundamental to pharmacology and toxicology, as tissue PK often offers a more accurate representation of drug activity at the site of action compared to plasma PK. MSI drug ion images are typically integrated with histological features from H&E stained tissue, with IHC enabling more selective protein target association.2 Furthermore, MSI enables precise quantification of drug distribution across entire tissue sections or specific regions of interest, often guided by histological features.3
Instrumentation advancements have expanded MSI’s applicability to include, for example, both multicellular studies and the ability to work at single cell resolution. These improvements in sensitivity and spatial resolution facilitate drug toxicity studies at relevant dosing regimens, reinforcing the role of MSI in understanding drug interactions within tissues and supporting informed decision making in drug development.
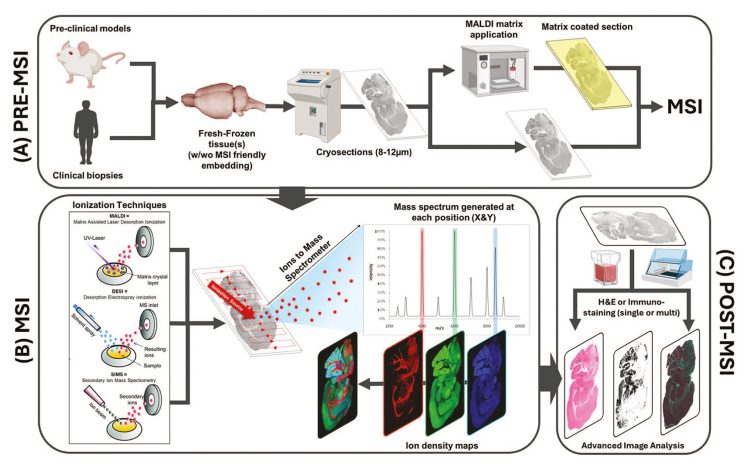

Figure 1: MS Imaging workflow9
Integrating MSI into toxicological investigations
Incorporating MSI into toxicological studies requires careful planning and collaboration across multidisciplinary teams, including toxicology, histopathology, drug metabolism (DMPK), bioanalysis and quality assurance. Effective integration ensures meaningful insights into drug distribution and toxicity.
The first step in study planning is determining the MSI limit of detection (LOD) for the parent drug and its metabolites. Retrospective studies may be constrained by prior necropsy timing and tissue availability, as formalin-fixed samples are often unsuitable due to drug diffusion or loss. In completed studies, comparing the MALDI MSI LOD with plasma PK data can help assess the feasibility. Prospective studies offer greater flexibility, allowing for optimised necropsy timing and flash-freezing of tissues for MSI analysis with input from study toxicologists and histopathologists.
The first step in study planning is determining the MSI limit of detection (LOD) for the parent drug and its metabolites.
MSI also complements tissue homogenate liquid chromatography-MS (LC-MS) analysis, which provides overall drug concentrations but cannot provide information about the spatial distribution within tissues. By integrating MSI with histopathology, clinical chemistry and DMPK data, researchers can develop mechanistic hypotheses of toxicity, improving decision making and reducing unnecessary follow-up studies.
For good laboratory practice (GLP) studies, project teams may consider freezing select tissue blocks for MSI under a non-GLP protocol. Aligning MSI study design with the 3R principles (replacement, reduction and refinement) ensures efficient use of animal studies while maximising the value of toxicological data. Strategic tissue collection allows for retrospective MSI analysis if toxicity concerns arise, minimising costs while preserving critical insights into drug safety.
Targeted drug delivery and therapeutic development
MSI offers significant advantages for those developing small molecule therapeutics. For instance, Cheng et al. (2022)4 combined MSI with in-life positron emission tomography (PET) imaging and ex vivo MALDI MSI biodistribution studies to create a comprehensive preclinical dataset for PK/PD modelling. This multimodal approach enhances our understanding of drug distribution and efficacy, and minimises systemic exposure and associated toxicity of the API.4
Similarly, Groseclose et al. (2022)5 used MSI to map specific lipids in skin, sebaceous gland and hair follicles, linking lipid distribution and biological functions, and aiding the development of lipid-based therapeutics. Furthermore, Yang et al. (2024)6 demonstrated MSI’s ability to distinguish biliary toxicants by revealing distinct PKs within hepatocytes and bile duct cells, informing the development of safer alternatives. These studies highlight MSI’s role in providing detailed spatially resolved data crucial for optimising drug efficacy and safety, and advancing therapeutic development.
MSI in emerging therapeutic modalities
Enhanced sensitivity and broader therapeutic applications are advancing the field of MSI. Innovations like single-cell analysis, the use of complex in vitro models and improved IHC integration (MALDI-IHC) are expanding its capabilities, particularly with regard to investigating biomarkers, metabolomics, lipidomics and glycomics.5 As MSI integrates with spatial multiomics, it will increasingly allow researchers to map the therapeutic impact on biological systems, informing drug safety and efficacy.6
MSI also plays a key role in advancing oligonucleotide therapeutics by enabling the identification of modifications and metabolites, ensuring stability and efficacy.7 In immune therapies, MSI helps monitor immune-cell distribution and off-target effects, optimising therapeutic strategies and improving patient outcomes.8
A positive impact
MALDI MSI is proving to be a transformative tool in toxicology and therapeutic development, enabling precise spatial imaging of APIs and metabolites within tissues. Multiomics MSI studies provide crucial insights into drug pharmacology and toxicity, supporting the development of innovative therapies including chimeric antigen receptor (CAR) T-cell treatments, oligonucleotide therapeutics, antibody drug conjugates (ADCs) and ribonucleic acid (RNA) therapeutics.
The technique offers significant benefits in preclinical translation, including early toxicity detection, insights into drug-tissue interactions and optimised dosing strategies. By improving early-stage decision making and reducing follow-up studies, MALDI MSI can help reduce risk and lead to better decisions as researchers strive for next-generation therapeutic options.
References
[1]. Castellino S, Lareau NM, Groseclose MR. The Emergence of Imaging Mass Spectrometry in Drug Discovery and Development: Making a Difference by Driving Decision Making. J. Mass Spectrom. 2021, 56 (8), e4717. https://doi.org/10.1002/jms.4717.
[2]. Xie F, Gales T, Ringenberg MA, et al. Characterizing the Distribution of a Stimulator of Interferon Genes Agonist and Its Metabolites in Mouse Liver by Matrix-Assisted Laser Desorption/Ionization Imaging Mass Spectrometry. Drug Metab. Dispos. 2024, 52 (11), 1181–1186. https://doi.org/10.1124/dmd.122.001076.
[3]. Barry JA, Groseclose MR, Castellino S. Quantification and Assessment of Detection Capability in Imaging Mass Spectrometry Using a Revised Mimetic Tissue Model. Bioanalysis 2019, 11 (11), 1099–1116. https://doi.org/10.4155/bio-2019-0035.
[4]. Cheng S-H, Groseclose MR, Mininger C, et al. Multimodal Imaging Distribution Assessment of a Liposomal Antibiotic in an Infectious Disease Model. J. Control. Release 2022, 352, 199–210. https://doi.org/10.1016/j.jconrel.2022.08.061.
[5]. Yagnik G, Liu Z, Rothschild KJ, Lim MJ. Highly Multiplexed Immunohistochemical MALDI-MS Imaging of Biomarkers in Tissues. J. Am. Soc. Mass Spectrom. 2021, 32 (4), 977–988. https://doi.org/10.1021/jasms.0c00473.
[6]. Zhang H, Lu KH, Ebbini M. Mass Spectrometry Imaging for Spatially Resolved Multi-Omics Molecular Mapping. npj Imaging 2024, 2, 20. https://doi.org/10.1038/s44303-024-00025-3.
[7]. van der Vloet L, Barbier Saint Hilaire P, Bouillod C, et al. How Can MSI Enhance Our Understanding of ASO Distribution? Drug Discovery Today 2025, 30 (1), 104275. https://doi.org/10.1016/j.drudis.2024.104275.
[8]. Bindi G, Monza N, Santos de Oliveira G, et al. Sequential MALDI-HiPLEX-IHC and Untargeted Spatial Proteomics Mass Spectrometry Imaging to Detect Proteomic Alterations Associated with Tumor-Infiltrating Lymphocytes. J. Proteome Res. 2024, Article ASAP. https://doi.org/10.1021/acs.jproteome.4c00914.
[9]. Vezzali E, Becker M, Romero-Palomo F, et al. European Society of Toxicologic Pathology—Pathology 2.0 Mass Spectrometry Imaging Special Interest Group: Mass Spectrometry Imaging in Diagnostic and Toxicologic Pathology for Label-Free Detection of Molecules—From Basics to Practical Applications. Toxicologic Pathology. 2025 Feb 4
About the authors


Steve Castellino received his BA in Chemistry from California State University, Long Beach, and his PhD in Organic Chemistry from the University of California, Riverside. His graduate research focused on the structural characterisation and synthesis of marine natural products. He went on to complete postdoctoral studies in Professor Gary Keck’s laboratory at the University of Utah, where he investigated mechanisms of stereoselective reactions.
Steve later accepted a faculty position in the Department of Chemistry at North Dakota State University. His research programme focused on elucidating the structure of Lewis acid complexes involved in stereochemical transformations.
In 1993, he joined Rhône-Poulenc, where he led the discovery spectroscopy group. Four years later, he moved to the drug metabolism group at GlaxoWellcome. During his 23-year tenure at GSK, he held various scientific and leadership roles, including Director of DMPK, where he oversaw drug metabolism characterisation. He also led the MALDI Imaging MS group, responsible for quantitative tissue biodistribution studies of drug candidates across both discovery and development phases.
Steve was the founding President of the Imaging MS Society. Since retiring from GSK, he has continued to contribute to the field as President of Xenovista LLC, Chief Scientific Officer at GlycoPath, and Principal Investigator at Bruker Glycobiology Solutions.


Stefan Foser brings together a strong academic background with nearly two decades of experience in the pharmaceutical and diagnostics industries. He holds a PhD in Microbiology and has earned academic qualifications from the Universities of Basel (Switzerland) and Mannheim (Germany). In addition to nearly twenty publications and patents, he also holds an Executive MBA from the University of St Gallen, Switzerland.
Stefan has held pivotal roles at leading life science companies including Roche and Siemens Healthineers. He currently serves as Vice President, Global Pharma, at Bruker Daltonics, where he leverages his deep sector knowledge to advance innovative solutions for the pharmaceutical industry.
Related topics
Drug Discovery, Drug Discovery Processes, Imaging, Mass Spectrometry, Toxicology, Translational Science
Related organisations
Bruker Daltonics