Anti-linker antibodies: a universal key for CAR-T detection
Posted: 19 September 2025 | Drug Target Review | No comments yet
Every new CAR-T needs a new detection tool – until now. Anti-linker antibodies could change how researchers develop and track these therapies.

Chimeric antigen receptor T-cell (CAR-T) therapies are moving beyond haematological malignancies into solid tumours and autoimmune diseases. On 01 June 2025, The Lancet published the world’s first randomised controlled trial of CLDN18.2-specific CAR-T therapy in gastric cancer, led by Professor Lin Shen’s group at Peking University. The study reported a significant increase in progression-free survival and a 44 percent improvement in overall survival.
Meanwhile, early clinical results are highlighting CAR-T’s promise in autoimmune disorders such as systemic lupus erythematosus and multiple sclerosis.
Despite these advances, CAR-T development remains limited by the challenge of reliably detecting CAR expression. Each new CAR design requires bespoke detection reagents, a process that is costly, time-consuming and variable.
The detection dilemma
CAR expression levels are critical for both preclinical validation and clinical monitoring. The CAR-positive rate directly influences target recognition, cytotoxic potency and therapeutic durability. Monitoring CAR expansion and persistence in vivo provides vital insights into treatment response, relapse risk and immunogenicity.
Currently, flow cytometry using recombinant antigens such as CD19 or BCMA is the standard for detecting CAR-T therapies. This method is target-specific and not suitable for new CAR constructs. Other approaches include Protein L, which binds only certain antibody light chains; polyclonal Fc-binding antibodies, which may cross-react with patient IgG; and anti-idiotype reagents, which recognise unique CAR regions but are difficult to develop. Each method has practical limitations that can slow CAR-T development.
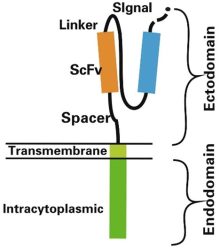

Schematic of CAR structure showing ectodomain, transmembrane region and endodomain. Source: Zhang et al. 2017
Anti-linker antibodies: a universal strategy
A new solution may now be at hand. Sino Biological has introduced anti-Whitlow 218 linker rabbit monoclonal antibodies, designed to recognise the Whitlow 218 linker sequence (GSTSGSGKPGSGEGSTKG). Since this linker is incorporated into nearly 90 percent of single-chain variable fragment (scFv)-based CAR constructs, the antibodies enable detection independent of the CAR’s antigen target.
This universal detection strategy offers several advantages:
- Broad applicability: detects any CAR with the Whitlow 218 linker, regardless of antigen specificity
- Flexible conjugation: available with FITC (fluorescein isothiocyanate) or PE (phycoerythrin), common flow cytometry dyes, with AF488, AF647, APC and biotin options in development.
- Exceptional specificity: minimal non-specific binding observed in peripheral blood mononuclear cells (<0.5 percent)
- In vitro and in vivo use: suitable for transfection efficiency assays and dynamic monitoring of CAR-T expansion post-infusion
- Accelerated timelines: eliminates the need for custom reagent development, reducing cost and complexity.
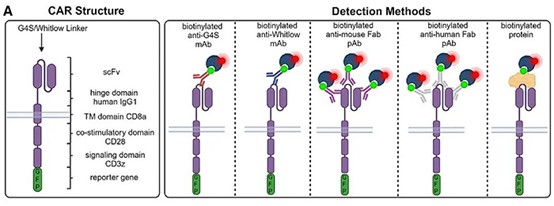

An image to illustrate different CAR detection technologies. Source: Schindler et al. 2024
Implications for CAR-T development
The Whitlow 218 linker, first described in 1993, promotes proper folding of antibody fragments and is widely used in scFv-based CAR constructs. Sino Biological’s antibodies target this conserved sequence, enabling universal CAR detection.
For researchers, this innovation means faster development cycles, more consistent data and reduced reliance on bespoke anti-idiotype reagents. For clinicians, it offers the potential for standardised monitoring across CAR-T therapies targeting diverse antigens from CD19–BCMA–Claudin18.2 and beyond.
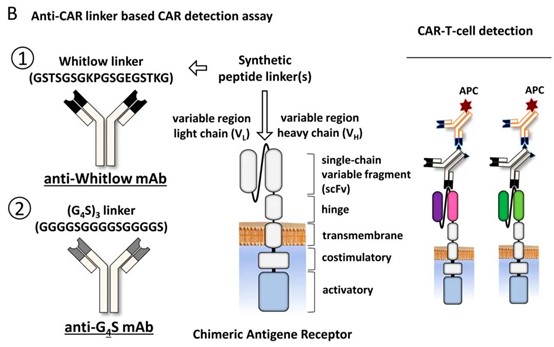

Technical principle of anti-CAR linker-based CAR detection assay. Source: Grahnert et al. 2024
As CAR-T therapies continue to diversify into oncology and autoimmunity, universal tools such as anti-linker antibodies may prove essential in accelerating the path from bench to bedside.
For more information, visit the Sino Biological’s CAR linker page.
References
- Zhang C, Liu J, Zhong JF, Zhang Y. Engineering CAR-T cells. Biomark Res. 2017;5:22. doi:10.1186/s40364-017-0102-y.
- Schindler K, Ruppel KE, Müller C, Koehl U, Fricke S, Schmiedel D. Linker-specific monoclonal antibodies present a simple and reliable detection method for scFv-based CAR NK cells. Mol Ther Methods Clin Dev. 2024 Aug 22;32(3):101328. PMID: 39286335; PMCID: PMC11403257.
- Grahnert A, Seiffert S, Wenk K, Schmiedel D, Boldt A, Vucinic V, Merz M, Platzbecker U, Klemann C, Koehl U, Friedrich M. Evaluation of anti-CAR linker mAbs for CAR T monitoring after BiTEs/bsAbs and CAR T-cell pretreatment. Biomedicines. 2024 Jul 24;12(8):1641. PMID: 39200107; PMCID: PMC11351819.
- Wang Z, Liu Y, Chen X, Zhao H, Sun J, Li Y, et al. CAR-T therapy dilemma and innovative design strategies for next generation. Cell Death Dis. 2025;16(5):454. doi:10.1038/s41419-025-07454-x.
Related topics
Analysis, Antibodies, Assays, Autoimmune disease, Cancer research, Cell Therapy, Chimeric Antigen Receptors (CARs), Drug Discovery, Drug Discovery Processes, Immunology, Immunotherapy, Monoclonal Antibody, Oncology, T cells
Related conditions
Gastric cancer, Multiple Sclerosis, solid tumours, Systemic Lupus Erythematosus
Related organisations
Sino Biological