Ubiquitous pyrogens and 3D cancer models
Posted: 11 March 2024 | Bjorn Veragauwen (Rousselot) | No comments yet
A recent study has highlighted the impact of endotoxins on the accuracy of three-dimensional (3D) cancer models. In this Q&A, Dr Bjorn Vergauwen reveals details and key findings of the research.

A collaboration between the University of Twente in the Netherlands and Rousselot has revealed the dynamics between biomaterial purity and the fidelity of 3D cancer models. Their joint study illuminated the profound impact of endotoxins on the accuracy and efficacy of these models, marking a significant breakthrough in cancer research. Through experimentation, contrasting research-grade gelatine, inherently infused with endotoxins, with an ultra-low endotoxin-level gelatine, they revealed that the presence of endotoxins significantly distorts the predictive power of 3D in vitro breast cancer immune models. This investigation underscores the imperative for researchers to consider endotoxin levels in their design of 3D cancer models, offering a pathway towards more reliable and clinically relevant research outcomes.
What were the key findings of the study regarding the impact of endotoxins on 3D breast tumour immune cancer models?
Bjorn Vergauwen (BV): The study has shown for the first time that the accuracy and predictive value of 3D in vitro breast cancer models is significantly impacted by the presence of endotoxins.1
Research-grade gelatine with naturally high endotoxin levels was used for gelatine methacrylate (GelMA) synthesis in the lab and compared with an ultrapurified GelMA with low endotoxin levels. Both GelMAs were used as a 3D cell culture medium in an in vitro model for breast cancer, containing both cancer cells and immune cells.
The in vitro model was used to evaluate the therapeutic effectiveness of novel breast cancer immunotherapies. However, endotoxin contamination in the lab-produced GelMA triggered immune cell populations to differentiate into an inflammatory phenotype. This made the in vitro model insensitive to the effects of the novel immunotherapy agents.
Overall, this research highlights the severity of endotoxicity in cancer models and emphasises the importance of choosing the right gelatine to achieve accurate 3D models.
How did the level of endotoxins in gelatines and methacryloyl gelatines (GelMA) affect the crosstalk between macrophages and cancer cells in the 3D models in the study?
BV: The researchers studied the impact of endotoxins on 3D breast cancer immune models, looking at both the effect on the crosstalk between macrophages and cancer cells and the effect on the measured efficacy of immunotherapies.
High-endotoxin gels measured significant gene inhibition and reduced the crosstalk between macrophages and cancer cells, making the macrophages less responsive. In contrast, the low-endotoxin biomaterial allowed significant upregulation of genes involved in the crosstalk between macrophages and cancer cells and demonstrated successful intercellular communication between the two cell types.
Can you elaborate on the specific ways in which high levels of endotoxins influenced the inflammatory reaction in immune cells (macrophages) within contaminated models?
BV: The standard gels created a more artificial inflammatory environment than the purified gels. In the biomaterial with high endotoxin levels, macrophages were 2.16 times larger and produced three times the amount of nitrous oxide, indicating a strong inflammatory response to the bioink.
Endotoxins also impacted the reliability of therapeutic outcomes. The high-endotoxin environment artificially increased the measured efficacy of the therapy that was designed to inhibit the expression of anti-inflammatory markers, while in the therapy designed to induce pro-inflammatory markers it seemed to show non-efficacy.
Why is it important to consider endotoxins in the design of 3D in vitro cancer models?
BV: Researchers should be aware of the potential interfering effects of endotoxins in their 3D models, which could lead to the misinterpretation of results, or in the worst case, the failing of in vivo experiments.
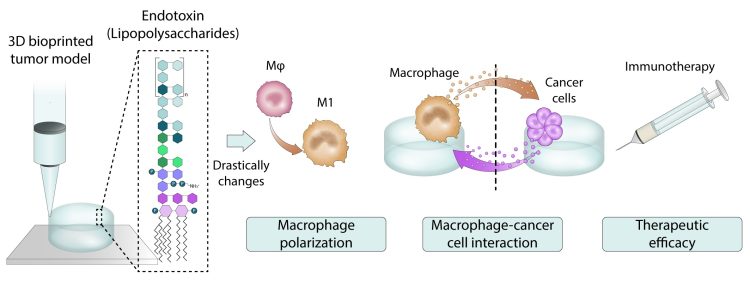

Figure 1. The presence of endotoxins in biomaterials can negatively impact the credibility of 3D models.
Endotoxins, or lipopolysaccharides – large, highly immunogenic molecules that form the major component of the outer membrane of Gram-negative bacteria – are a known problem in the world of biomaterials and medical devices. Even at low levels, endotoxins can pose a serious health risk. When exposed to endotoxins, the immune system initiates an inflammatory response that can lead to tissue inflammation, increased sensitivity to other allergens, and the risk of fatal shock.2
Using purified biomaterials can help to ensure that the biomaterial does not negatively impact the survival of cells, disrupt the crosstalk between different cells or alter the functionality or performance of evaluated therapeutics.
The study suggests that highly purified gelatine biomaterials can contribute to a more accurate representation of the safety and potency of novel therapeutics. How can this finding potentially reduce the reliance on animal testing in the field of cancer drug testing?
BV: Recent changes in legislation and increased ethical considerations,2 together with the recent success of 3D models like organoids and organs-on-a-chip, are driving a shift towards cutting-edge alternatives to animal-based research.
3D printing allows innovators to produce complex structures and intricate organ models, which can be used to study diseases and test new therapies in a more realistic and efficient manner. By reducing the need for animal models, 3D printing not only improves ethical considerations but can also reduce the costs and time associated with animal studies.
While there has been an exponential growth in the development of 3D models, the effects of ingrained endotoxins in many commercially available biomaterials have been overlooked and remain unreported. This study unravels how the impurity of endotoxins can mislead the outcome of macrophage-targeted immunotherapies, as tested in the 3D model.
To ensure that new treatments can progress safely to clinical trials, the impact of endotoxins and choice of biomaterial should be considered right from the start of research.
About the author


Scientific Director for Product and Process Development at Rousselot
He leads R&D projects focused on unlocking the full clinical potential of gelatin. His expertise lies in the biophysical and biochemical principles underlying gelatin’s behaviour, which he applies to various applications in the regenerative and pharmaceutical space.
Related topics
Immuno-oncology, Immunology, Targets
Related organisations
Rousselot








