Optimising CRISPR gene editing of hard-to-transfect cells
Posted: 12 April 2024 | Dr Georges Muller (SEED Biosciences Ltd) | No comments yet
CRISPR has transformed gene editing, but still presents challenges in hard-to-transfect cells, such as pluripotent stem cells and primary cells.1 The key to obtaining successful transfection in these cells lies in innovative workflows. Here Georges Müller, CEO and cofounder of SEED Biosciences, shares his perspective on why focusing on editing a single cell, rather than bulk cells, is a pivotal strategy to optimise CRISPR delivery.
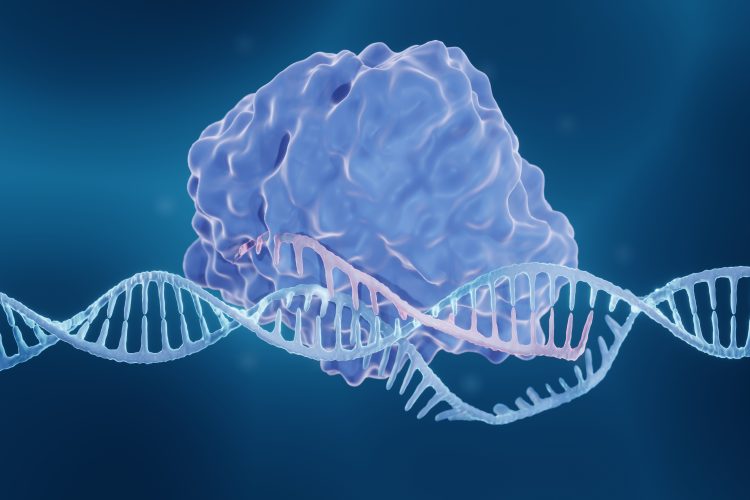
Delivery of ribonucleoprotein (RNP) into cells is an essential factor for successful CRISPR gene editing. However, this is difficult to guarantee using traditional CRISPR gene editing methods, especially in hard-to-transfect cells. The standard CRISPR technique involves gathering a group of cells and then electroporating them, using short high-voltage pulses to overcome the barrier of their cell membranes. This allows bulk transfection of ribonucleoprotein (RNP) into the cells and then hopefully, nuclear translocation.
However, the traditional process can lack precision. Controlling the amount of RNP entering each cell is challenging, leading to uncertainty about the ratio of RNP that reaches any specific cell. This method of transfection is not uniformly distributed and achieving a 100% equal delivery is not guaranteed. Importantly for the researcher, there is no assurance that the RNP, once inside the cells, will reliably enter their nuclei. In hard-to-transfect cells, it is unlikely that this method will offer high transfection efficiencies. Additionally, post-transfection, the cells must undergo sorting, isolation, and subsequent cultivation, which can be a labour-intensive process.
New workflows in CRISPR gene editing
An alternative, more precise, approach involves isolating individual cells prior to transfection. This method has been developed by Cytosurge, and SEED Biosciences represents a step in this workflow.2 In this method, individual cells are seeded at the centres of multiple plates, and then the RNP is gently injected directly into the nucleus of each cell. This method provides careful control over the amount of RNP entering each cell, ensuring accurate measurement. The direct injection of RNP into the nucleus targets the specific site where it is needed, rather than broadly injecting it into the cell. Therefore, this meticulous method significantly improves the chances of achieving high transfection efficiencies in hard-to-transfect cells.
In addition, by adopting a gentle and precise approach, injecting directly into the nucleus means the utilisation of up to approximately 100 times less RNP compared to traditional methods. This not only benefits the project budget but also supports the quality of the cell editing process.
Once transfection has occurred, the cells can be grown to produce large monoclonal cell lines. Starting from a single cell rather than bulk cells eliminates the need for post-transfection cell sorting, saving hands-on time and ensuring 100 percent monoclonality. This holds particular significance, as it is a prerequisite for FDA approval in biotechnology that any cell clones must originate from a single-cell progenitor.
A considered approach
However, there are some considerations that need to be taken into account when using this method. For instance, it’s advisable to test the cells first, to ensure they can be grown to avoid finding out after transfection. Giving careful consideration to the cell isolation method is a crucial aspect of the entire process. For instance, the use of limiting dilution can be time-consuming and labour-intensive, often requiring two rounds to achieve satisfactory results.
The use of a single cell seeding robot eliminates the need for manual pipetting, significantly boosting throughput and enabling the seeding of five times more wells with viable cells. Furthermore, an automated system provides instant assurance of monoclonality, avoiding the need for additional equipment to check for this, which could introduce contamination risks, extra costs, and prolonged lead times. The precision of a single cell seeding robot ensures that each cell is accurately placed in the centre of the well, which is vital for the precise injection of RNP.
CRISPR has made a substantial impact on gene editing, improving precision and speeding up research. However, to utilise its potential in hard-to-transfect cells, innovative workflows are required. By isolating single cells prior to transfection and injecting RNP directly into the nucleus, research scientists can achieve high transfection efficiencies in hard-to-transfect cells — helping to bring CRISPR treatments to patients faster.
References
2 Rosen, H. (2023) ‘The Ins and Outs of Single Cell Gene Editing’, New Matter. [Podcast]. Available at: https://slas.buzzsprout.com/951535/12714008-the-ins-and-outs-of-single-cell-gene-editing-sponsored-by-molecular-devices (Accessed 24th January 2024).
About the author

Dr Georges Muller, CEO and cofounder of SEED Biosciences Ltd
Georges holds a master and a PhD degree in bioengineering from the Ecole Polytechnique Fédérale de Lausanne (EPFL).
He is the CEO and cofounder of SEED Biosciences Ltd, a spinoff from the EPFL. With its disruptive pipetting technology, SEED Biosciences is bridging the gap between the capabilities in liquid handling and the opportunities in single cell biology.
He is co-inventor of SEED Biosciences’ core technology called DISPENCELL and co-authored several patents and scientific articles that made the cover of SLAS Technology in 2020. He is the recipient of multiple awards for young entrepreneurs, including the Venture Leaders Prize given to top Swiss entrepreneurs. As a member of the Organizing Committee for the ESACT2017, he contributed to the success of this prestigious scientific conference in the field of Animal Cell Technology.
Georges has been an active member of the SLAS’ entrepreneurship and innovation scenes. He was awarded with the Ignite Award at SLAS 2019. A year later in San Diego, he was enlisted in the Top 10 Finalists for the Innovation Award. In 2022, SEED Biosciences received its very first and prestigious New Product Award.
Related topics
CRISPR, Genome Editing, Stem Cells
Related organisations
SEED Biosciences Ltd