Aggregate formation for monoclonal antibodies
Posted: 5 September 2018 | Tony Edge | No comments yet
Workflows are generated around the analysis of large protein-derived therapeutics. The use of different analytical techniques is necessary to support drug development and testing, and thus increase generation of these new therapies. One of the areas that is most critical to this process is the determination of aggregates.

An individual protein has three levels of structure denoted by the primary amino acid chain, the relatively short intramolecular interactions due to hydrogen bonding, and the tertiary structure, which is generated as the protein folds in on itself over a larger range. There is, however, a fourth type of structure that is often associated with proteins, which is the intermolecular bonding of proteins though relatively weak electrostatic and hydrogen bonding interactions. This results in the formation of two types of aggregates: structures (dimers/trimers, etc) that are not stable and exist in a dynamic equilibrium – and in these cases pressure and temperature can cause a shift in the equilibrium; and irreversibly-formed aggregates that are stable and result in the formation of subvisible particles. This can result in the epitopes from the biological system being exposed to a different confirmation of the antibody. These subvisible particles are of increasing concern for the regulatory agencies due to their potential for causing unintended immunogenicity. It is therefore important to be able to detect and quantify the amount of aggregates that are formed during the manufacturing process.
Size exclusion chromatography
Size-based separations are a form of chromatography which, unlike the other chromatographic techniques mentioned in this article, separate molecules without any chemical interaction with the stationary phase. The concept was first postulated by Synge and Tiselius,1 with the first application being the analysis of proteins, demonstrated by Lindqvist and Storgårds.2 Size exclusion chromatography (SEC) uses an aqueous-based mobile phase making it an ideal technology with which to analyse proteins in a native state, since the use of organic solvents in HILIC and RP-LC results in the denaturation of protein structure.
It is, therefore, particularly useful to the biopharmaceutical industry for ensuring the purity of the biotherapeutic. During the manufacturing process, aggregation of the proteins within the cell culture can occur during several phases of the production process, including:
- product expression
- downstream processing
- Storage.
Aggregates found in monoclonal antibodies may cause an immune response and so accurate quantification is essential. One of the most popular approaches to determining the level of aggregation is using SEC.
The separation process in size exclusion chromatography is based on the hydrodynamic size or Stokes radius of the molecule,3 with smaller molecules having greater access to the pore structure and, as a result, eluting later in the chromatograph. One of the advantages of SEC is that the mobile phase – typically an aqueous buffer solution – allows the protein to exist in its native state, whereas other forms of chromatography that use organic solvents, can result in the protein denaturing, causing a change in the shape and consequently the Stokes radius of the protein. The stationary phase used is designed to be an inert material – typically, a hydrophilic coated polymer or silica-based material, with a narrow size distribution of pores. The inertness ensures that the separation is based solely on the size of the molecule and not on a combination of chemical interactions and size, which would make interpretation of the data very difficult.
The first peak to elute from an SEC column will be the largest components. Since these will be too large to enter into any of the pore structure, these components will elute in the same retention volume (V0) as the interstitial space between particles, or the exclusion limit. From here, other analytes will elute, with the retention time depending on the ability of the molecule to penetrate further into the accessible pore structure. The smallest molecules, including the solvent front, will elute at the end of the chromatograph, in a permeation limit, which represents the total accessible volume of solvent in both the interstitial space and the particle pores (Vtot). Since the process of retention is dependent on the hydrodynamic size of the molecule, it is possible to relate the retention times of individual analytes to their molecular mass as the mass of a molecule is related to the hydrodynamic radius.4
If the retention mechanism is purely based on the Stokes radii, then the retention time for an individual analyte is directly proportional to the log of the relative molecular mass5 for molecules that elute between the exclusion limit and molecules that can penetrate the entire pore network (Equation 1).
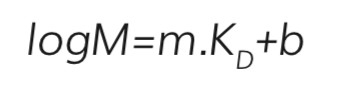
Where;
m and b are the slope and intercept of the linear part of the calibration line, and KD, the thermodynamic retention factor, is given by the following expression (Equation 2):
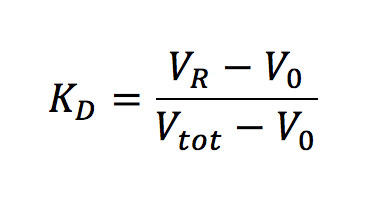
Where;
VR – retention volume of the analyte/protein
V0 – retention volume of the column
Vtot – total solvent volume of the column.
The variability in exact pore structure often leads to a degree of nonlinearity when the calibration line is constructed using real experimental data. Calibration plots are constructed using a series of known molecular weight/size samples, to allow identification of precise retention times for a particular molecular weight, MW. Obviously, information regarding the nature of the samples is required to allow a calibration range to be determined that covers the range of the samples being analysed. The calibration curve will provide an upper and lower MW limit over which the column can determine the molecular weight of the analytes.
The two key features of SEC are:
- Large pore volume, as this drives the separation, thus a larger column will provide a better separation. This is unlike the situation with other forms of chromatography where the column diameter should not affect the performance of the separation.
- An inert surface to ensure that the separation is driven solely by molecule size.
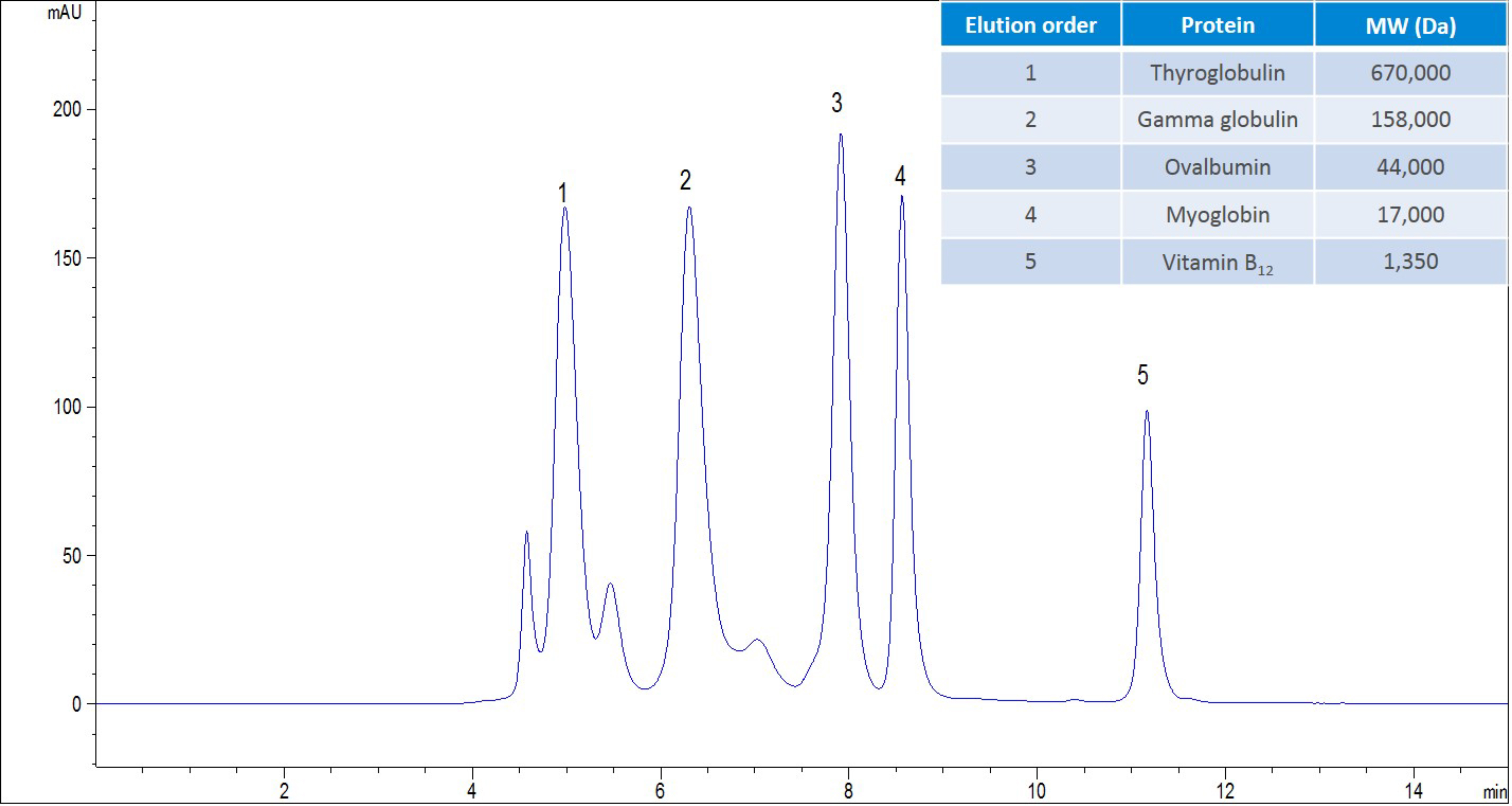
Figure 1. AdvanceBio SEC 2.7µm, 300 Å, 4.6 x 300 mm analysing gel filtration standard, (BioRad PN: 151-1901) with a flow rate of 350 µL/min, 150 mM sodium phosphate at pH 7.0.
Figure 1 shows an example chromatogram obtained from the analysis of a mAb, in this case an antibody drug conjugate. Resolution has been obtained between the ADC aggregate and the ADC monomer. The separation of these two components is an important quality control aspect, as it will enable the determination of efficacy and toxicity for the biotherapeutic and whether the separation meets the requirements of the industry, in this situation. The resolving power of SEC is not as good as other forms of chromatography that are used within the biopharmaceutical workflow, and this is due to the use of the pore volume to drive the separation. There are approaches that can be employed to improve the resolution, with the obvious approach being to reduce the particle size. However, this approach to improving the resolution creates two challenges:
- The increased pressure may result in protein configurational changes of the native protein
- The porous structure must be able to withstand the increase in pressure and there will be a point at which the structure of the particle is simply not strong enough to withstand the pressure.
Biomolecules often contain both hydrophilic and hydrophobic groups, which easily interact with traditional packing sorbents. These different moieties derived from the amino acid backbone, which comprises hydrophobic, hydrophilic, acidic and basic components. These different moieties can result in secondary interactions, which result in a shift in retention times, causing incorrect prediction of MW values. The secondary interactions also significantly impact peak shape, which will have a pronounced effect on the resolving ability of the separation process. Increasing the ionic strength/salt concentration can minimise polar interactions through electrostatic screening; however, this approach may also affect the form of the molecule being analysed and reduces the compatibility of the technique with some detectors. Hydrophobic interactions can be overcome by the addition of an organic component to the mobile phase; however, this can cause protein molecules to denature, which will alter their hydrodynamic size, and so this should be avoided if the native size of the protein is being determined.
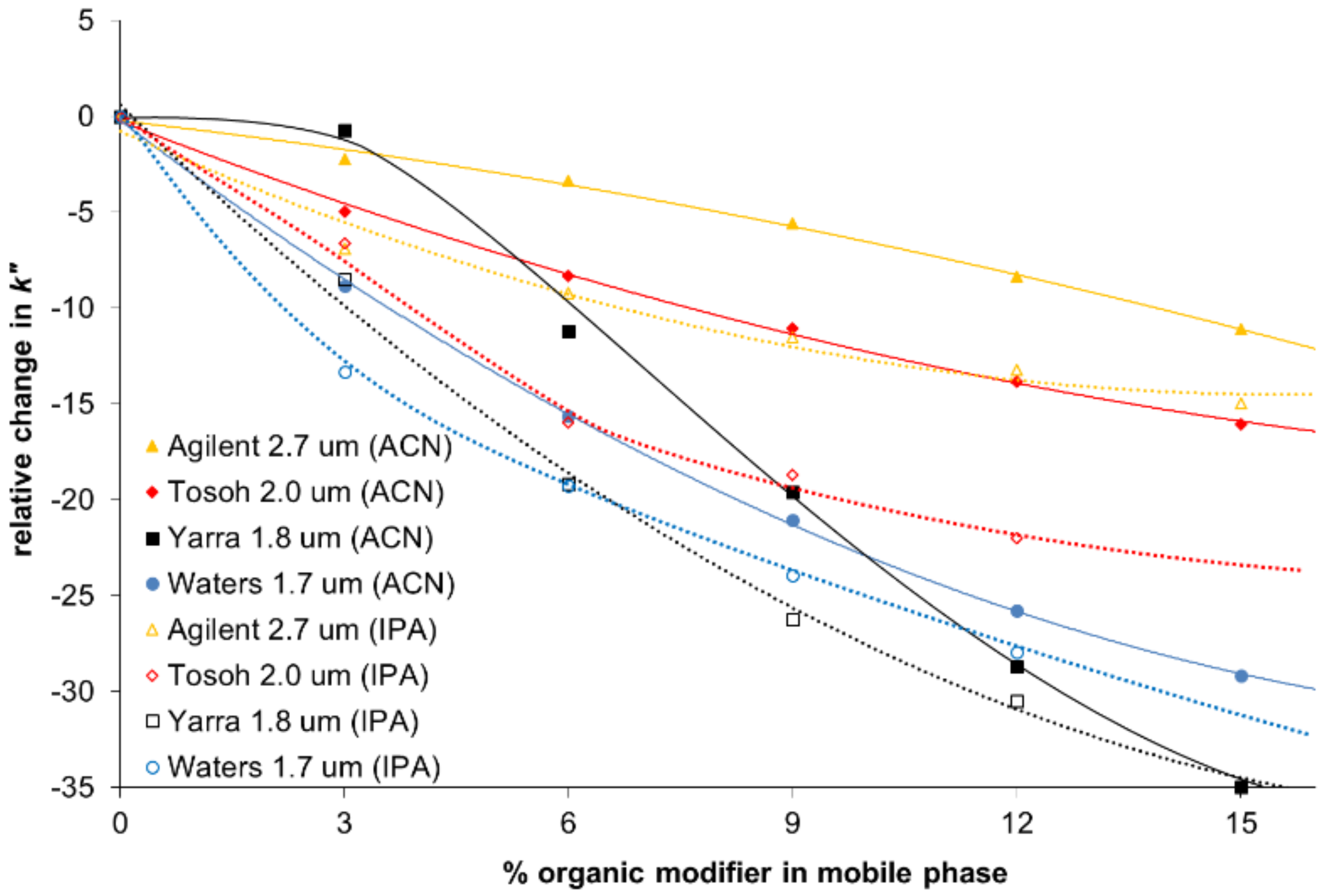
Figure 2
Relative change of zone-retention factor vs. organic modifier ipilimumab. Reprinted with kind permission from A. Goyon, A. Beck, O. Colas, K. Sandra, D. Guillarme, S. Fekete, Separation of protein biopharmaceutical aggregates using size exclusion chromatographic columns packed with sub-3 μm particles, A. Goyon, A. Beck, O. Colas, K. Sandra, D. Guillarme, S. Fekete, J. Chromatogr. A., 19 (2017) May 1498:80-89
A recent study by Goyon investigated these different nonspecific interactions on a range of SEC columns and demonstrated how this information can be used to compare the performance of a variety of columns.6 In this study hydrophobic interactions were investigated by monitoring the retention time of a range of mAbs with varying organic conditions. Increasing the organic modifier concentration should not alter the retention time and so shifts in retention time will indicate the presence of a hydrophobic interaction between the column and the test probe. One of the test components chosen was Ipilimumab, which is a very hydrophobic component and, as such, is sensitive to the amount of organic modifier in the system. The resulting relative decrease of the retention time was significant when increasing the content of ACN and IPA with all columns tested, with the largest change seen resulting in a shift of over 25% (Figure 2).
Conclusion
This article has provided an insight into the formation and analysis of aggregates. The process of analysis is focused on the use of size exclusion chromatography and this article has studied the theory associated with the separation process and also highlighted some of the issues that can cause increased retention between the analytes and the stationary phase. The use of additives in the mobile phase can reduce these secondary interactions; however, it can also result in the protein being affected so that the analytical system no longer records the correct size of the molecule. The use of higher resolution columns – achieved using smaller particles – can drive the chromatographic performance but even here, care must be taken to ensure that configurational changes in the protein do not affect data integrity.
References
- Synge R, Tiselius A. Biochemical J. 46 (1950) xli.
- Lindqvist B, Storgårds T. 175 (1955) 511-512.
- Atkins P. Physical Chemistry. 8 (2006) 766.
- Caltabiano AM, Foley JP, Barth HG. Chromatogr. A. 1437 (2016), 74-87.
- Yau WW, Ginnard CR, Kirkland JJ. Chromatogr. A. 149 (1978) 465-487.
- Goyon A,Beck A, Colas O, Sandra K, Guillarme D, Fekete S. Chromatogr. A. 19 (2017) May 1498:80-89.
BIOGRAPHY
Tony Edge is an R&D Manager at Agilent Technologies, heading a team of specialist scientists in developing next-generation stationary phases for HPLC. He has worked in both manufacturing and industry, having periods of employment at LGC, AstraZeneca, Thermo Fisher Scientific and latterly Agilent Technologies. In 2008 he was fortunate enough to be awarded the Desty memorial lecture for his contributions to innovating separation science, and in the same year also won a clinical excellence award from AstraZeneca. Tony’s current interests are centred on improving the extraction process and high-temperature chromatography. Tony was awarded an honorary fellowship at Liverpool University, where he lectures on separation science. He also lectures at Keele University on Management in Analytical Science and is the President of The Chromatographic Society in the UK as well as a contributing editor for the Chromatography Today magazine. Tony is also part of the Reid Bioanalytical conference organising committee, and a permanent member of the scientific committee for the International Symposium on Chromatography.
The rest of this content is restricted - login or subscribe free to access
 Thank you for visiting our website. To access this content in full you'll need to login. It's completely free to subscribe, and in less than a minute you can continue reading. If you've already subscribed, great - just login.
Thank you for visiting our website. To access this content in full you'll need to login. It's completely free to subscribe, and in less than a minute you can continue reading. If you've already subscribed, great - just login.
Why subscribe? Join our growing community of thousands of industry professionals and gain access to:
- quarterly issues in print and/or digital format
- case studies, whitepapers, webinars and industry-leading content
- breaking news and features
- our extensive online archive of thousands of articles and years of past issues
- ...And it's all free!
Click here to Subscribe today Login here
Related topics
Antibodies
Related organisations
Agilent Technologies - UK