Breast cancer cells invade by a physical mechanism
Posted: 15 November 2023 | Drug Target Review | No comments yet
Findings from using a 3D hydrogel to study how cancer cells physically tear the basement membrane offers promise for targeted treatment.
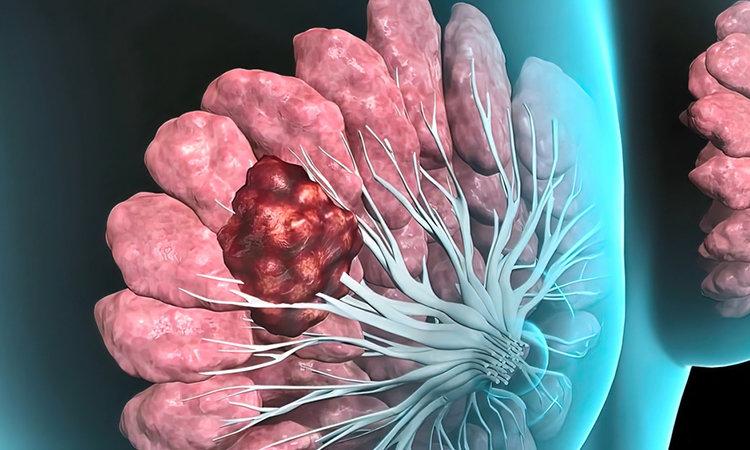

Most breast cancers begin in the lining of a breast milk duct and are very treatable if they remain there. However, once these cancers break through the basement membrane, a thin matrix around the duct, treatment becomes much more difficult.
New research by scientists at Stanford University has identified a novel physical mechanism used by breast cancer cells to become invasive. In addition to degradation of the basement membrane by established chemical methods, cancer cells also work together to physically deform and tear through the basement membrane barrier.
Dr Ovijit Chaudhuri, Associate Professor of Mechanical Engineering and Bioengineering, said: “When this invasion process has been studied, the focus has typically been on single cells.” He continued: “But what we know is that the invasion is actually collective in nature, involving groups of cells working together to penetrate through the basement membrane. Our work has elucidated how cells act together to break through the basement membrane, advancing our fundamental understanding of this critical transition in cancer progression.”
Mechanical forces
Previous research has demonstrated that individual cancer cells produce proteases, enzymes that degrade some of the basement membrane, and treatments inhibiting proteases have not been able to prevent cancer cells from doing this. Chaudhuri and his team believed there were other mechanisms at work, so they made a new model to study breast cancer and the basement membrane in three dimensions (3D).
“The current paradigm is that cells use chemicals to degrade through the basement membrane, but we show that this physical aspect is just as important.”
Dr Julie Chang, the paper’s lead author and former doctoral student in Chaudhuri’s lab, explained: “The current paradigm is that cells use chemicals to degrade through the basement membrane, but we show that this physical aspect is just as important.”
A 3D hydrogel mimicking the properties of breast tissue was designed with cultured cellular structures, named acini, that display features of a breast duct and are surrounded by their own basement membrane. The basement membrane was tagged with fluorescent markers so the team could observe and measure any deformation when cancerous cells interacted with it.
Surprising findings
Cancer cells within the acini swelled together which stretched the basement membrane. This stretching process thinned and weakened the basement membrane, enabling cancer cells near it to apply additional forces to open holes and escape through.
Postdoctoral researcher in Chaudhuri’s lab and co-lead author Dr Aashrith Saraswathibhatla, said “The cells are expanding and increasing their volume in unison, and then locally pulling to tear up the basement membrane.” He continued: “This collective volume expansion hasn’t been predicted before – no one was thinking about it.”
“This collective volume expansion hasn’t been predicted before – no one was thinking about it.”
The team determined that their findings were consistent with what has been observed in invasive breast cancer patients. Their colleagues at the University of Pennsylvania validated their results with computational modelling, confirming the physical forces involved could theoretically enable cells to escape the basement membrane.
Targeted treatment
Understanding the mechanical forces that cancer cells apply may result in new therapeutic strategies to block invasion and may help scientists decide which pre-invasive breast cancer patients have the highest likelihood of their cancer breaking out and spreading.
The researchers will investigate how cancerous cells physically interact with the surrounding breast tissue once they have escaped through the basement membrane. Further study of the basement membrane itself is also required, looking at both the mechanical and structural features, as some tumours become invasive whilst others do not.
“This global volume expansion mechanism represents a new insight into how the breast tumours become invasive, borne out of the development of a 3D culture model that allowed us to visualise the invasion process,” Chaudhuri said. “It highlights the emergent behaviours arising in groups of cells acting together that enable cancer invasion.”
This study was published in Nature Materials.
Related topics
Oncology, Therapeutics
Related conditions
Breast cancer
Related organisations
Pennsylvania University, Stanford University