Novel system developed to improve cancer detection
Posted: 24 August 2017 | Dr Zara Kassam (Drug Target Review) | No comments yet
Researchers have developed a proof-of-concept nano-system that dramatically improves the visualisation of tumours…
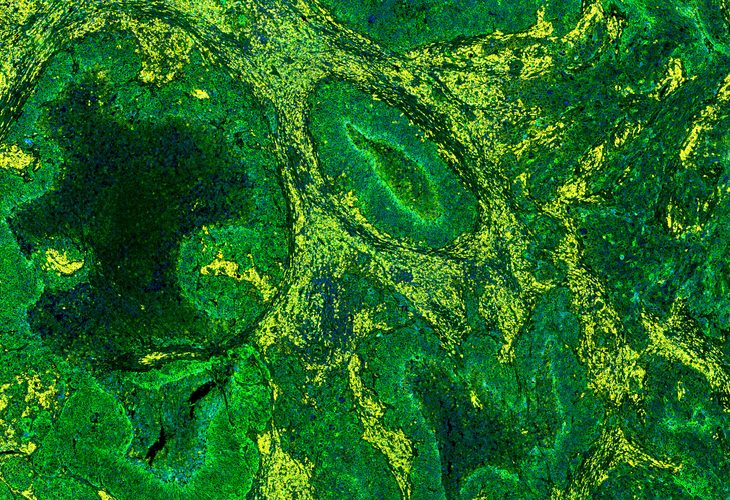

Researchers have developed a proof-of-concept nano-system that dramatically improves the visualisation of tumours, the platform achieves a five-fold increase over existing tumour-specific optical imaging methods.
The novel approach generates bright tumour signals by delivering “quantum dots” to cancer cells without any toxic effects.
“Tumour imaging is an integral part of cancer detection, treatment and tracking the progress of patients after treatment,” said Dr Kazuki Sugahara, Assistant Professor at Sanford Burnham Prebys Medical Discovery Institute (SBP). “Although significant progress has been made in the last two decades, better and more sensitive detection, such as the method we are developing, will contribute to more personalised and potentially more effective interventions to improve the clinical outcomes of cancer patients.”
The new method utilises quantum dots, QDs–tiny particles that emit intense fluorescent signals when exposed to light–and an “etchant” that eliminates background signals. The QDs are delivered intravenously, and some of them leave the bloodstream and cross membranes, entering cancer cells. Fluorescent signals emitted from excess QDs that remain in the bloodstream are then made invisible by injecting the etchant.
“The novelty of our nano-system is how the etchant works,” explains Dr Gary Braun. The etchant and the QDs undergo a “cation exchange” that occurs when zinc in the QDs is swapped for silver in the etchant. Silver-containing QDs lose their fluorescent capabilities, and because the etchant can’t cross membranes to reach tumour cells, the QDs that have reached the tumour remain fluorescent. Thus, the entire process eliminates background fluorescence while preserving tumour-specific signals.
The method was developed using mice harbouring human breast, prostate and gastric tumours. QDs were actively delivered to tumours using iRGD, a tumour penetrating peptide that activates a transport pathway that drives the peptide along with bystander molecules–in this case fluorescent QDs–into cancer cells.
To our knowledge, this is the first in vivo example of a background-destroying etchant being used to enhance the specificity of imaging,” says Dr Sugahara. “We are encouraged that we were able to achieve a tumour-specific contrast index (CI) between five- and ten-fold greater than the general cut-off for optical imaging, which is 2.5.”
“Moving forward we will focus on developing our novel nano-system to work with routine imaging tests like PET scans and MRIs. In our studies with mice, we use optical imaging, which isn’t always practical for humans,” said Dr Sugahara.
Related topics
Imaging, Magnetic resonance images (MRI), Oncology, Positron emission tomography (PET)
Related conditions
Cancer
Related organisations
Dr Gary Braun, Sanford Burnham Prebys Medical Discovery Institute
Related people
Dr Gary Braun, Dr Kazuki Sugahara