Novel material for regenerative medicine can control cell immune response
Posted: 6 November 2019 | Victoria Rees (Drug Target Review) | No comments yet
Researchers have created a material that can manipulate the immune response and be used as a regenerative medicine therapy.

Researchers have created a new material that they suggest has promising uses in regenerative medicine. According to the team, damaged tissues and blood vessels could be recovered with their development.
The study was conducted by researchers at Tomsk Polytechnic University, Russia jointly with the University of Montana, US.
The scientists used a biodegradable polycaprolactone polymer to create three-dimensional (3D) scaffolds. The thin polymer fibres are interwoven with each other in different directions and their unique material makes them more flexible and affordable than other scaffolds used in regenerative medicine.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
The scaffolds were built by the researchers using a method of electrospinning, which produces very thin fibres from a polymer solution under the electric field. Through this, the team were able to introduce inhibitors into the polymer structure. These were two compounds: 11H-indeno [1,2-b] quinoxaline-11-on oxime (IQ-1) and 11H-indeno [1,2-b] quinoxaline-11- on O-(O-ethylcarboxymethyl) oxime (IQ-1E).
“Inhibitors suppress or slow down physiological and physicochemical processes. They affect enzymes. To do this, the enzyme and the inhibitor must fit together like a lock and a key,” explained Ksenia Stankevich, author of the article and engineer of the Laboratory for Plasma Hybrid Systems.
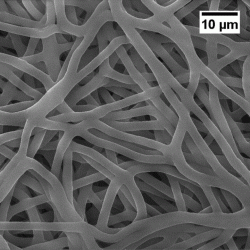

This is the scaffold structure (credit: Tomsk Polytechnic University).
The inhibitors are able to affect the JNK group of enzymes, which are responsible for the inflammatory process. Therefore, the material can control the entire subsequent immune reaction chain, which the researchers demonstrated on human blood and cell lines.
These inhibitors are released over a long period of time, for a prolonged effect, due to the natural degradation of the polymer. Additionally, it degrades to biocompatible 6-hydroxycaproic acid, which is recycled by human body, say the researchers.
“Eventually, our scaffolds could be used to recover damages of soft tissues and blood vessels. The polycaprolactone has all suitable mechanical properties. For instance, it can reduce the negative consequences after a heart attack and stroke,” the researchers said.
The results were published in ACS Biometerials Science & Engineering.
Related topics
Regenerative Medicine, Research & Development, Targets
Related organisations
Tomsk Polytechnic University, University of Montana
Related people
Ksenia Stankevich