Cancer immunotherapies may be improved with early immune response
Posted: 5 December 2019 | Rachael Harper (Drug Target Review) | No comments yet
A new mechanism has been reported for detecting foreign material during early immune responses which may help detect elusive cancers.

Researchers and colleagues from the University of Illinois at Chicago, US have shown a new mechanism for detecting foreign material during early immune responses which could be used to inform the development of immunotherapies.
“There are proteins in the cell that await the presence of foreign material,” said Marlene Bouvier, senior author and UIC professor of microbiology and immunology at the College of Medicine. “One protein, called endoplasmic reticulum aminopeptidase 1, or ERAP1, is programmed to find foreign material, like viral and bacterial proteins and break it into smaller parts, also known as peptides.
“Another protein – major histocompatibility complex class I, or MHC I – is programmed to attach itself to a foreign peptide and move it to the cell’s surface. With the foreign peptide outside the cell, immune cells can recognise and destroy the infected cell.”
However sometimes a foreign peptide, once bound to MHC I, remains in the cell, Bouvier said. This happens when the foreign material is not broken down to a small enough size or is too long.
Bouvier and colleagues used X-ray crystallography and mass spectrometry to show that ERAP1 can cut extra-long peptides even after they have bound to MHC I.
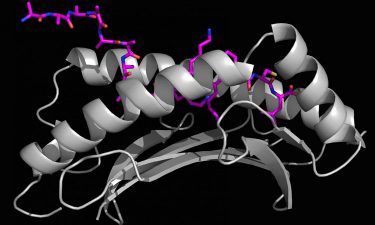

X-ray crystallography generated image of a long foreign peptide (purple) being partially held inside MHC I protein’s surface groove (grey) (credit: Marlene Bouvier).
“X-ray crystallography allowed us to determine three-dimensional (3D) structures to see how these longer peptides bound to the MHC I groove with high resolution,” Bouvier continued. “Using an ERAP1 enzymatic assay with mass spectrometry gave us the ability to show, for the first time, that ERAP1 can trim peptides bound to MHC I. These tools allowed us to develop a model of this new immune response mechanism.”
Bouvier said this new information may help researchers leverage ERAP1 to fight infections and cancer.
“This research can have major implications for immunotherapies,” said Bouvier. “For example, cancer cells do not always present enough peptides to be labeled as ‘foreign’ – allowing the cancer cells to replicate and grow. But if you have a way to manipulate how ERAP1 generates cancer peptides, then you can hopefully skew the peptide repertoire that is presented on the cell surface in our favour. This is the most translational application of our research.”
The report was published in the Journal of Biological Chemistry.
Related topics
Crystallography, Imaging, Immuno-oncology, Immunology, Immunotherapy, Mass Spectrometry, Oncology
Related conditions
Cancer
Related organisations
University of Illinois at Chicago (UIC)
Related people
Marlene Bouvier