GPR3 identified as a new target for Alzheimer’s disease treatment
Posted: 15 October 2015 | Victoria White
Researchers have determined that the protein GPR3 might play an important role in alleviating the cognitive deficits of ‘amyloid plaques’…
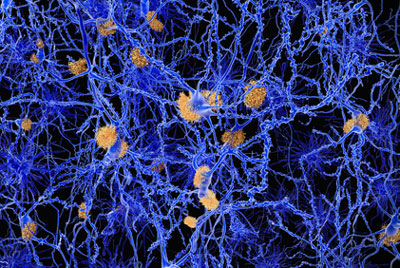

Researchers have determined that the protein GPR3 might play an important role in alleviating the cognitive deficits of ‘amyloid plaques’.
The identification of GPR3 as an Alzheimer’s disease (AD) therapeutic target provides an important new perspective in AD drug development.
AD is characterised by a gradual degeneration of the brain networks that are involved in memory and cognition.A pathological hallmark of AD is the accumulation of protein fragments between nerve cells in the brain. These protein fragments are created by secretases, enzymes that cleave proteins into smaller pieces. One of the fragments that is produced following cleavage by the γ-secretase is the amyloid-β peptide, which aggregates to form amyloid plaques. The accumulation of amyloid plaques in the brains of Alzheimer’s disease patients leads to the gradual degeneration of brain networks and disruption of mental function.
GPR3 research could eventually lead to AD drug discovery
Amantha Thathiah, VIB/KU Leuven, who led the research, said, “We discovered that G protein-coupled receptor 3 (GPR3), a protein expressed in the brain, plays a significant role in the generation of amyloid- β peptides and accumulation of amyloid plaques. Our research indicates that the absence of GPR3 alleviates the cognitive decline and reduces amyloid pathology in multiple disease-relevant models. These studies identify GPR3 as a therapeutic target for AD and provide a significant level of validation necessary for the future of AD drug discovery.”
AD mouse models are important tools to study AD progression. However, no mouse model fully captures the pathological changes in AD patients. Consequently, validation of AD therapeutic targets in multiple models is required prior to advancing to clinical research.
Amantha Thathiah added, “Although the loss of GPR3 reduced amyloid burden and improved cognition in four AD mouse models, we also evaluated the GPR3 expression in post-mortem brain tissue from two cohorts of AD patients. These studies revealed that GPR3 expression is elevated in a subset of AD patients and is associated with disease progression.”
These studies provide a significant preclinical validation for GPR3 and set the bar for therapeutic validation in the development of AD drug targets.
Related topics
Drug Discovery, Drug Targets, Enzymes, Target Validation
Related conditions
Alzheimer’s disease