Experimental peptide targets glioblastoma’s most resilient cells
Posted: 3 June 2025 | Drug Target Review | No comments yet
An experimental peptide from Virginia Tech may offer a new way to stop glioblastoma from coming back by disrupting the cancer’s treatment-resistant core.

Scientists at Virginia Tech’s Fralin Biomedical Research Institute have developed a peptide drug that may offer a promising new approach to tackling glioblastoma – an aggressive and notoriously treatment-resistant brain cancer.
Published in Cell Death and Disease, the team revealed that their lab-designed molecule, JM2, can selectively kill glioblastoma stem cells, the very cells that drive recurrence after surgery, chemotherapy and radiation.
“Glioblastoma stem cells can adapt easily to both their environment and treatment,” said Samy Lamouille, assistant professor at the Fralin Biomedical Research Institute. “These cells can lie dormant, and at some point, they reawaken and then rebuild the tumour. It’s critical to find a way to target this population of cancer cells.”
Glioblastoma is the most common form of malignant brain tumour. It grows rapidly, is difficult to fully remove surgically, and almost always returns. Standard therapies provide only modest improvements in survival – the median time after diagnosis is just over 14 months.
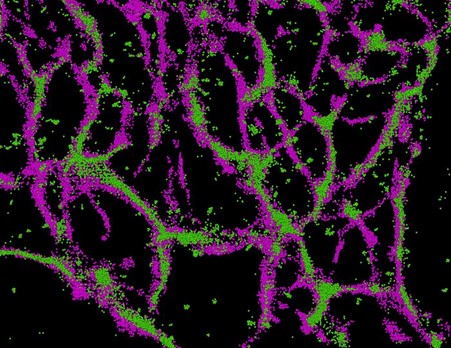

Super-resolution microscopy reveals connexin 43 (green) closely associated with microtubules (magenta) in glioblastoma stem cells – a link that may help the cells resist treatment and drive tumour spread. Credit: Samy Lamouille / Virginia Tech
Disrupting cancer’s survival systems
Lamouille’s team focused their efforts on connexin 43, a protein known for its role in enabling cells to communicate. In glioblastoma, however, this protein appears to wear two hats – sometimes helping to suppress tumour growth, and other times supporting it.
Using super-resolution microscopy, Lamouille and Associate Professor James Smyth discovered that connexin 43 in glioblastoma stem-like cells binds closely to structures called microtubules, which help the cells maintain their shape, divide, and move.
That discovery led to JM2 – a peptide designed to mimic the microtubule-binding domain of connexin 43 and disrupt its function in glioblastoma stem cells.
JM2 was also toxic specifically for these particular cells, leaving healthy brain cells unharmed.
“When we tested JM2 in glioblastoma stem-like cells, that was the most exciting moment,” said Lamouille. “Not only did that efficiently disrupt connexin 43 interaction with microtubules, but JM2 was also toxic specifically for these particular cells, leaving healthy brain cells unharmed.”
The peptide’s effect was surprisingly potent.
“The JM2 peptide had a killing effect by itself. That was unexpected,” said Rob Gourdie, developer of JM2. Gourdie, who first created the peptide at the Medical University of South Carolina, is now a co-founder of Acomhal Research Inc., which has licensed JM2 for further development.
Moving toward clinical potential
In preclinical tests, JM2 not only disrupted cancer stem cell survival in lab experiments but also significantly slowed tumour growth in animal models. The peptide achieved this without compromising other crucial functions of connexin 43 – a key concern in developing targeted therapies.
While human trials are still ahead, the team is now exploring targeted delivery systems – including biodegradable nanoparticles and viral vectors to get JM2 directly to the tumour.
If successful, JM2 could represent a new class of peptide therapies that do what standard treatments cannot: stop glioblastoma at its root.
This study was published in Cell Death and Disease.
Related topics
Cancer research, Drug Targets, Microscopy, Molecular Biology, Molecular Targets, Stem Cells, Targets
Related conditions
Brain cancer, Glioblastoma
Related organisations
Acomhal Research Inc., Virginia Tech