Two-faced protein both inhibits and activates B cell receptor signalling
Posted: 6 April 2022 | Ria Kakkad (Drug Target Review) | No comments yet
New research could point towards a way to restore immune function in patients with immune disorders involving B cell signalling deficiencies.

Researchers from the Tokyo Medical and Dental University (TMDU), Japan found that CD22, a crucial molecule in B cell signalling, switches from an inhibitory role to an activating role when B cell receptor (BCR) signalling is compromised due to a genetic defect that causes an immune disorder. The study was recently published in Science Signaling.
Contact between BCRs and foreign invaders prompts B cells to make antibodies, and CD22 inhibits BCR signalling to keep B cells from inappropriately releasing antibodies. Interestingly, previous research suggests that this inhibition is regulated by binding of CD22 to other factors expressed on the same cell. In contrast, a protein called CD45 is a main activator of BCR signaling, and defects in the gene encoding CD45 cause an immunodeficiency syndrome.
“CD45 normally enhances BCR signalling,” explained Chizuru Akatsu, lead author on the study. “When CD45 is missing in laboratory cell lines, BCR signalling is dramatically decreased; however, signalling is not affected as severely in mice when CD45 is missing, which suggests that there is some kind of compensatory mechanism at work.”
To investigate the relationship between CD22 and BCR signalling restoration in the absence of CD45, the researchers disrupted the binding of all interaction partners of CD22 either continuously or for a short time and looked at the effect this had on BCR signalling.
“The results were entirely unexpected,” said Takeshi Tsubata, senior author. “Acute disruption of binding between CD22 and its ligands did not affect the restoration of BCR signalling in B cells lacking CD45, whereas continuous disruption of this binding resulted in markedly less BCR signalling recovery.”
As it turns out, the cells in which signalling was restored expressed unusually high levels of BCR, which accounted for their ability to continue functioning relatively normally. BCR signalling occurs at low levels even in the absence of stimulation by foreign antigens, and this low-level steady state signalling is required for B cell development and survival. As BCR is an endogenous ligand of CD22, continuous CD22 binding to its ligands facilitates inhibition of steady-state BCR signalling by CD22. If BCR signalling is compromised by a defect such as CD45 deficiency, steady state signalling is markedly reduced by the signalling defect together with the signal inhibition by CD22; therefore, only B cells that express high levels of BCR survive. Through this mechanism, CD22 paradoxically restores BCR signalling in immune-deficient B cells.
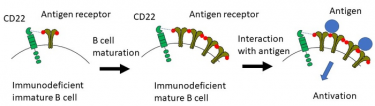

When antigens interact with the antigen receptor on B cells, B cells undergo activation and differentiation to antibody-producing cells (plasma cells). Defects in signaling through the antigen receptor cause immunodeficiency. In this study, the authors showed that the function of immunodeficient B cells is restored by increasing the amount of antigen receptors during development of mature B cells. This process requires interaction of CD22 with its cis-ligands
[Credit: Department of Immunology, TMDU].
“What is really interesting about this result is that it could point toward a way to restore immune function in patients with immune disorders involving B cell signalling deficiencies,” concluded Akatsu.
Given that B cells and immunoglobulins are present, though in greatly reduced numbers, in immunodeficient patients with defects in BCR signalling, CD22 may be a useful treatment target. Activating CD22 could help restore B cell function in patients with deficiencies such as X-linked agammaglobulinemia.
Related topics
Antibodies, Gene Testing, Immunology, Immunotherapy, Protein
Related conditions
Immunodeficiency, X-linked agammaglobulinemia
Related organisations
Tokyo Medical and Dental University (TMDU)
Related people
Chizuru Akatsu, Takeshi Tsubata