Novel targets identified for treatment of schistosomiasis
Posted: 24 July 2023 | Izzy Wood (Drug Target Review) | No comments yet
Researchers from Brazil discovered that survival of the parasitic worm that cause the disease schistosomiasis, depends on expression of a specific type of RNA. In animal trials, inhibition of the molecule interrupted the infection.
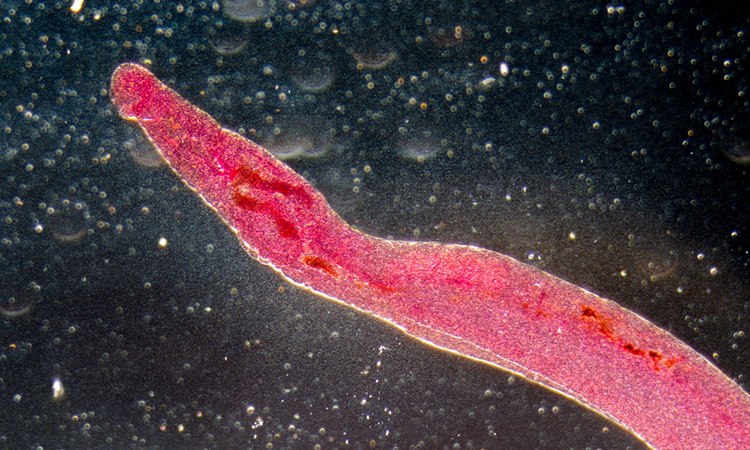

Researchers at the Butantan Institute in São Paulo, Brazil, have made a significant breakthrough in the fight against schistosomiasis. The team has discovered a way to disrupt the reproductive cycle of the parasitic worms (Schistosoma mansoni) responsible for the disease, potentially paving the way for a new therapeutic approach. Their findings, published in PLOS Pathogens, highlight the essential role of long non-coding RNAs (lncRNAs) in the parasite’s survival, making them a promising target for future treatment strategies.
Schistosomiasis, caused by parasitic worms, affects around 200 million people worldwide and has remained a significant health challenge for decades. One of the peculiarities of these worms is their requirement for male and female adults to remain paired together throughout their lives for successful reproduction. Female worms can produce up to 3,000 eggs daily, with roughly half reaching the host’s gut or bladder, while the remainder causes inflammation and liver cirrhosis when carried via the bloodstream to the liver and spleen, ultimately leading to mortality.
The research team, led by Professor Sergio Verjovski-Almeida of the University of São Paulo and the Butantan Institute, embarked on a Thematic Project supported by FAPESP to explore the role of lncRNAs in human cancer and schistosomiasis. Despite being a major component of human cells’ RNA (97 percent), lncRNAs were previously overlooked due to their lack of known functions. However, emerging evidence from cancer research indicated their potential significance in disease development.
“We found that lncRNAs play a crucial role in maintaining homeostasis in the schistosomiasis-causing parasite, making them attractive targets for therapeutic interventions,” said Murilo Sena Amaral, co-corresponding author of the study and a researcher at Butantan Institute’s Cell Cycle Laboratory.
To achieve this breakthrough, the researchers analysed data from public repositories to identify specific lncRNAs in S. mansoni that were most or least expressed when male and female worms were paired or separated. Based on the results, they selected three lncRNAs as candidate therapeutic targets.
The researchers first conducted in vitro assays, exposing pairs of male and female worms to a double-stranded RNA molecule capable of targeting specific lncRNAs. The treatment led to a separation of the parasites, reduced viability, and inhibited egg release, ultimately resulting in their death.
Next, the team performed experiments in mice infected with S. mansoni By injecting the same double-stranded RNA into the animals’ bloodstream, they successfully decreased the target lncRNA in the parasites, leading to their death and a decrease in the viability of their eggs.
Schistosomiasis is classified as a neglected tropical disease, and for over 40 years, the only available drug to treat it has been praziquantel. However, the drug has its limitations, including reports of resistant worms, highlighting the urgent need for alternative treatment options. The researchers’ study offers hope for a potential new drug that could silence the expression of lncRNAs in the parasite, effectively eliminating them from the host’s bloodstream.
The discovery marks a significant step forward in schistosomiasis research, holding the promise of a novel therapeutic approach to combat this debilitating disease. With further research and development, this groundbreaking study could lead to improved treatments that will make a difference in the lives of millions affected by schistosomiasis worldwide.
Related topics
Animal Models, Disease Research, In Vivo, RNAs
Related conditions
Schistosomiasis
Related organisations
Butantan Institute
Related people
Murilo Sena Amaral, Professor Sergio Verjovski-Almeida