Japanese squirrels develop human hereditary diseases with ageing
Posted: 14 September 2023 | Drug Target Review | No comments yet
Researchers at Tokyo University of Agriculture and Technology, led by Dr Tomoaki Murakami, have uncovered a previously undocumented disease, fibrinogen Aα-chain amyloidosis, highly prevalent in Japanese squirrels, shedding light on its parallels with the human condition. Their findings offer new insights into amyloidosis and its potential implications for medical research.

Dr Tomoaki Murakami and his research team at Tokyo University of Agriculture and Technology have made a discovery regarding fibrinogen Aα-chain amyloidosis, an ailment previously unreported in animals other than humans. Their study reveals a high prevalence of this condition in Japanese squirrels (Sciurus lis), exploring its comparative pathology with human cases and emphasising the importance of Japanese squirrels in advancing our understanding of fibrinogen Aα-chain amyloidosis. The findings of this research were published on August 8th in the Journal of Pathology.
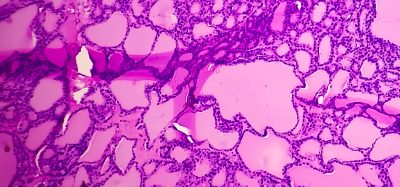
Amyloidosis is a group of diseases characterised by the deposition of amyloid, a misfolded protein, in various organs. Fibrinogen Aα-chain amyloidosis is an inherited disorder where the fibrinogen Aα-chain, a protein involved in blood coagulation, forms amyloid deposits in the renal glomeruli, ultimately leading to renal failure. Though first reported in 1993, this disease lacks a definitive treatment due to the limited number of patients and numerous unknowns regarding its pathogenesis. In this study, it was discovered that fibrinogen Aα-chain amyloidosis is remarkably common in Japanese squirrels, opening the door to a comparative analysis of this disease’s pathogenesis in humans.
The research team conducted a comprehensive histopathological examination of the organs from 38 captive Japanese squirrels that had passed away at five different zoos in Japan between 2018 and 2022. Surprisingly, 29 of these cases (76.3 percent) displayed systemic amyloidosis, characterized by severe glomerular amyloid deposition. Susumu Iwaide, the first author and a graduate student in the Laboratory of Veterinary Toxicology at TUAT, expressed astonishment, noting that such a high incidence of amyloidosis in one animal species is rare. The researchers employed mass spectrometry-based proteomic analysis and immunohistochemistry, identifying fibrinogen Aα-chain as the precursor protein. Interestingly, the amyloid deposits were exclusively found in the glomeruli of the affected kidneys, mirroring the human condition, as stated by Iwaide.
Further analysis utilising mass spectrometry revealed the accumulation of approximately 100 amino acids in the C-terminal region of the fibrinogen Aα-chain within amyloid deposits, closely resembling the human disease. Gene analysis showed no mutations in the amyloid-forming region when comparing squirrels with and without amyloidosis. Statistically, a significant association between amyloidosis and aging was observed. Additionally, some affected individuals were closely related to wild-protected squirrels. Iwaide concluded that the reduced genetic diversity resulting from zoo breeding is not the cause of fibrinogen Aα-chain amyloidosis in Japanese squirrels but rather an age-related condition inherent to the species.
In human fibrinogen Aα-chain amyloidosis, mutations occur in the gene responsible for the amyloid-forming region in individuals who develop the disease, leading to alterations in the amino acid sequence. This underscores the importance of the C-terminal region of the fibrinogen Aα-chain in maintaining protein stability. Given that the amyloid deposition pattern in the kidneys of Japanese squirrel’s mirrors that in humans, it is suggested that fibrinogen Aα-chain shares a common mechanism of amyloid formation across various animal species.
Related topics
In Vitro, Proteomics
Related organisations
Tokyo University of Agriculture and Technology (TUAT)
Related people
Dr Tomoaki Murakami (Tokyo University)