Treatment for polycystic kidney disease shows promising results
Posted: 13 September 2019 | Rachael Harper (Drug Target Review) | No comments yet
A potential treatment for polycystic kidney disease has shown positive results in animal testing.

A potential treatment for polycystic kidney disease, which causes the kidneys to swell with multiple cysts, has shown promising results in animal testing, with approximately 50 percent reduction in kidney size in afflicted mice following treatment.
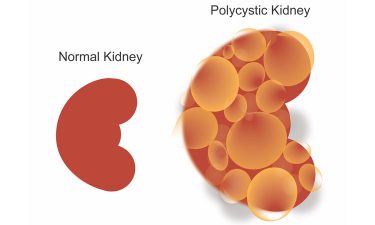

Polycystic kidney disease causes the kidneys to swell with multiple cysts. The new treatment showed a reduction of 50 percent kidney size in animal testing (credit: UT Southwestern).
The new treatment for autosomal dominant polycystic kidney disease (ADPDK) cooperatively developed at UT Southwestern, US and Regulus Therapeutics Inc, US showed no evidence of toxicity in animals or in human cell tests, according to the study. It is preferentially delivered to kidneys rather than the liver after being administered.
“We earlier showed that levels of a tiny RNA fragment called microRNA-17 are increased in models of ADPKD,” said Dr Vishal Patel, Associate Professor of Internal Medicine at UT Southwestern and senior author of the study. “MicroRNA-17 interferes with the normal function of other, beneficial RNAs, causing kidney cysts to grow. RGLS4326, as the new drug is called in development, works by blocking the harmful microRNA-17.”
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
Early Phase I clinical trials for the new treatment for polycystic kidney disease began last year. The US Food and Drug Administration (FDA) has asked for additional toxicity information from animal testing before human trials can move to the next step, Dr Patel said.
The study was published in Nature Communications.
Related topics
Clinical Trials, Drug Delivery, Drug Development, Drug Discovery, Formulation, RNAs
Related conditions
autosomal dominant polycystic kidney disease
Related organisations
Regulus Therapeutics Inc, US Food and Drug Administration (FDA), UT Southwestern
Related people
Dr Vishal Patel