Scientists significantly reduce errors in CRISPR-Cas9 genome editing system
Posted: 1 December 2015 | Victoria White | No comments yet
The team from the Broad Institute and the McGovern Institute for Brain Research found that mutations in three amino acids dramatically reduced “off-target” cuts…
Researchers at the Broad Institute and the McGovern Institute for Brain Research have engineered changes to the CRISPR-Cas9 genome editing system that significantly cut down on “off-target” editing errors.
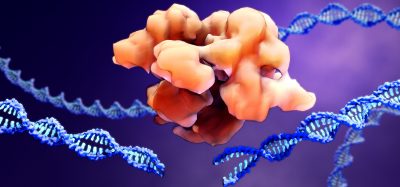
The CRISPR-Cas9 system works by making a precisely targeted modification in a cell’s DNA. The protein Cas9 alters the DNA at a location that is specified by a short RNA whose sequence matches that of the target site. While Cas9 is known to be highly efficient at cutting its target site, a major drawback of the system has been that, once inside a cell, it can bind to and cut additional sites that are not targeted. This has the potential to produce undesired edits that can alter gene expression or knock a gene out entirely, which might lead to the development of cancer or other problems. However, the researchers report that changing three of the approximately 1,400 amino acids that make up the Cas9 enzyme from S. pyogenes dramatically reduced “off-target editing” to undetectable levels in the specific cases examined.
Feng Zhang and his colleagues used knowledge about the structure of the Cas9 protein to decrease off-target cutting. DNA, which is negatively charged, binds to a groove in the Cas9 protein that is positively charged. Knowing the structure, the scientists were able to predict that replacing some of the positively charged amino acids with neutral ones would decrease the binding of “off target” sequences much more than “on target” sequences.
The newly-engineered enzyme is called eSpCas9
Zhang’s team found that mutations in three amino acids dramatically reduced “off-target” cuts. For the guide RNAs tested, “off-target” cutting was so low as to be undetectable.
The newly-engineered enzyme, which the team calls “enhanced” S. pyogenes Cas9, or eSpCas9, will be useful for genome editing applications that require a high level of specificity. The Zhang lab is immediately making the eSpCas9 enzyme available for researchers worldwide. The team believes the same charge-changing approach will work with other recently described RNA-guided DNA targeting enzymes, including Cpf1, C2C1, and C2C3.
Zhang concluded, “We hope the development of eSpCas9 will help address some of those concerns, but we certainly don’t see this as a magic bullet. The field is advancing at a rapid pace, and there is still a lot to learn before we can consider applying this technology for clinical use.”
Related topics
Amino Acids, CRISPR, Enzymes
Related organisations
Broad Institute