Single-cell approach generates RNA atlas of breast cancer tissue
Posted: 17 May 2021 | Victoria Rees (Drug Target Review) | No comments yet
Researchers have measured the gene expression of healthy and cancerous single cells from breast tissue, creating an ‘RNA atlas’.
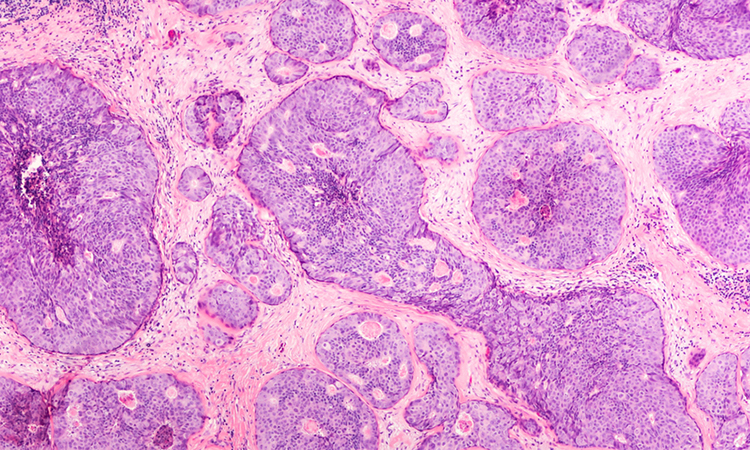

Researchers have documented the diversity of cells in the human breast, explaining the relationship between healthy breast cells and breast cancer cells. The study was conducted at the Walter and Eliza Hall Institute, Australia.
The research measured the gene expression of single cells taken from healthy women and cancerous breast tissue, including tissue carrying a faulty BRCA1 gene. This enabled the researchers to create an ‘RNA atlas’ that details the different cells found in these tissues.
The single-cell technologies used enabled the research team to isolate more than 340,000 individual cells from breast tissues donated by women and men and to measure the expression of different genes in these cells.
“Different types of breast cancer arise from distinct precursor cells. However, breast cancer development can be impacted by other cells within the breast,” said Professor Jane Visvader, one of the lead researchers. “This atlas provides a high-resolution view into the various cell types that make up breast tissue in different states and a blueprint for studying changes that lead to breast cancer.”
According to the researchers, this atlas will enable researchers to better understand the different cell types that constitute breast tissue and how these change during the development of cancer.
“Complex bioinformatic analysis was crucial for documenting the complex cellular landscape. For example, we found that the composition of a particular subset of cells in the breast was altered by menopause – a period of significant hormonal change within the body,” said Dr Yunshun Chen, one of the researchers.
“This will be an invaluable resource for breast cancer researchers around the world. Our research also has important implications for not only understanding how breast cancers arise but also how cells in the surrounding environment contribute to their development, spread and response to treatment,” said Visvader.
The study was published in EMBO Journal.
Related topics
Next-Generation Sequencing (NGS), Oncology, RNAs, Sequencing
Related conditions
Breast cancer
Related organisations
Walter and Eliza Hall Institute
Related people
Dr Yunshun Chen, Professor Jane Visvader