Next-gen malaria vaccine created using mapped parasite protein
Posted: 11 August 2025 | Drug Target Review | No comments yet
Australian scientists have, for the first time, visualised a malaria parasite protein complex–a discovery that has led to a new mRNA vaccine capable of blocking the parasite’s reproduction in mosquitoes and potentially halting transmission.


Malaria is one of the world’s deadliest infectious diseases, causing over 600,000 deaths annually. Despite progress in treatment and prevention, stopping transmission between mosquitoes and humans has still not been achieved.
But now for the first time, Australian scientists have captured the detailed structure of a crucial protein complex in malaria parasites – revealing a promising new target for vaccines aimed at stopping the spread of the disease.
Using advanced cryo-electron microscopy, researchers at the Walter and Eliza Hall Institute (WEHI) visualised a protein complex essential for malaria parasite fertilisation inside mosquitoes. Their study has led to the development of a new mRNA vaccine candidate that blocks the parasite’s reproduction in mosquitoes – potentially breaking the cycle of transmission before it infects humans.
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
Visualising malaria’s reproductive machinery
Scientists have long known that two proteins on the malaria parasite surface – Pfs230 and Pfs48/45 – are critical for the parasite’s transmission. However, exactly how these proteins interact was not understood.
“Our structural biology approach was the key. Using cryo-electron microscopy, we were able to visualise the full fertilisation complex directly from the parasite – not a lab-made version,” said lead researcher Dr Melanie Dietrich, a WEHI postdoctoral fellow. “This gave us a clear picture of how this fertilisation complex really looks in nature and revealed a previously unknown region that’s crucial to the process, unlocking a powerful new vaccine target.”
The scientists used these findings to create a vaccine that showed promise in targeting these contact points.
From structural insight to vaccine innovation
Unlike many studies that rely on artificially produced proteins, this research purified the fertilisation complex directly from malaria parasites – a technically challenging step that ensures the structure reflects its true form in nature.
The team identified critical contact points where Pfs230 and Pfs48/45 bind. Removing these contact points in genetically modified parasites stopped fertilisation and blocked transmission – highlighting an effective vaccine target.
Building on this discovery, the researchers designed a next-generation mRNA vaccine – formulated in collaboration with the mRNA Core facility at the Monash Institute of Pharmaceutical Sciences (MIPS).
In preclinical trials, this vaccine triggered strong antibody responses that recognised the parasite and blocked its transmission in mosquitoes by up to 99.7 percent.
“Drawing on experience through mRNA Core, the MIPS team shifted focus to tackle a new challenge in malaria vaccination. The success of the malaria vaccine program illustrates the versatility of mRNA technology, which has many applications beyond the COVID vaccines,” Professor Colin Pouton from MIPS. “It was particularly rewarding to work on this project with the WEHI team, co-located in the Parkville precinct, where shared expertise has helped drive a new approach to malaria prevention.”
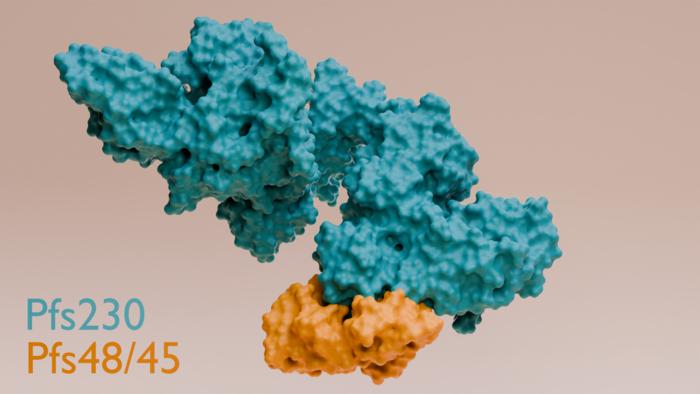

A depiction of proteins Pfs230 and Pfs48/45 bound together. This binding process is crucial for the malaria parasite’s ability to fertilise and spread. Credit: WEHI
Targeting a vulnerable stage in the parasite’s life cycle
The malaria parasite undergoes a “population bottleneck” inside the mosquito. While abundant in humans, only a small fraction develops into the sexual forms capable of fertilisation in mosquitoes. Targeting the parasite at this stage can significantly reduce transmission.
Transmission-blocking vaccines like this target the parasite inside mosquitoes, when its population is smallest and most vulnerable – providing a strategic advantage in controlling the disease.
Towards a multi-stage strategy for malaria elimination
The researchers believe that their mRNA vaccine is part of a broader multi-stage approach that targets the parasite both inside mosquitoes and within the human host.
“The ability to design, formulate and test vaccine candidates within a single research ecosystem has accelerated the path from discovery to preclinical validation,” Professor Tham said.
By combining transmission-blocking vaccines with others targeting the parasite’s blood or liver stages in people, scientists hope to build a comprehensive defence that could greatly reduce malaria and bring eradication closer.
Related topics
Disease Research, Drug Discovery, Drug Discovery Processes, Drug Targets, Structural Biology, Vaccine development
Related conditions
Malaria
Related organisations
Walter and Eliza Hall Institute (WEHI)