Advancing gene editing: the role of lipid nanoparticles in CRISPR delivery
Posted: 8 October 2025 | the Acuitas Therapeutics team (author details below) | No comments yet
CRISPR therapies depend on delivery and lipid nanoparticles are emerging as a more flexible and scalable option than viral vectors.

Gene editing through clustered regularly interspaced short palindromic repeats (CRISPR) associated proteins has unlocked new possibilities in treating disease. However, since these CRISPR-based therapeutics are primarily being developed as nucleic acid-based medicines, delivery strategies are proving to be just as important for enabling CRISPR medicines as the gene-editing technology itself.
For much of the earlier work, CRISPR medicines were primarily delivered through viral vectors, leveraging the virus’ ability to deliver genetic material. Yet viral methods of CRISPR delivery – particularly adeno-associated viruses (AAVs) to deliver DNA – have inherent limitations, such as modest payload capacity and patients’ immune responses to the viral capsid.
Delivering RNA that encodes CRISPR is an option gaining momentum in the field of gene editing. However, this method faces its own challenges.
Delivering RNA that encodes CRISPR is an option gaining momentum in the field of gene editing. However, this method faces its own challenges. Introduced without a delivery vehicle, nucleic acids such as RNA have poor cellular uptake, are susceptible to degradation in the bloodstream and may trigger immune responses. For these reasons, a reliable delivery platform is necessary.
Biomarkers aren’t just supporting drug discovery – they’re driving it
FREE market report
From smarter trials to faster insights, this report unpacks the science, strategy and real-world impact behind the next generation of precision therapies.
What you’ll unlock:
- How biomarkers are guiding dose selection and early efficacy decisions in complex trials
- Why multi-omics, liquid biopsy and digital tools are redefining the discovery process
- What makes lab data regulatory-ready and why alignment matters from day one
Explore how biomarkers are shaping early drug development
Access the full report – it’s free!
Lipid nanoparticles (LNP) have emerged as the preferred non-viral CRISPR delivery platform for in vivo applications. LNPs have been proven in other therapeutic and vaccine applications and have demonstrated several key advantages including their ease of manufacturing, relatively low immunogenicity and their transient nature – all of which make LNPs a particularly attractive option for delivery.
LNP fundamentals for CRISPR delivery
Composition and mechanism
LNPs are small, homogeneous particles (with a mean diameter of 50-120nm) composed of lipids designed to encapsulate and deliver nucleic acid payloads. They generally consist of three key components: ionisable lipids, structural lipids (such as phospholipid and cholesterol) and steric lipids such as PEG-lipids.
LNPs are small, homogeneous particles (with a mean diameter of 50-120nm) composed of lipids designed to encapsulate and deliver nucleic acid payloads.
Ionisable lipids – such as ALC-0315™, the primary lipid component of the COMIRNATY® vaccine – play key roles in the function of LNPs to deliver RNA into target cells. Their pH-responsive properties enable them to have a positive charge during formulation manufacturing to facilitate the efficient encapsulation of nucleic acids, but be neutral in physiological environments, minimising toxicity. However, as LNP is endocytosed, the progressive acidification results in protonation of the ionisable lipid and eventual association and destabilisation of the endosomal membrane. This allows efficient release of the RNA cargo into the cell’s cytoplasm, where it performs its function.
The polyethylene glycol (PEG) lipids also play an important role in the performance of LNP. PEG lipids primarily control physical stability during manufacturing and storage, but sheds from the LNP when administered in vivo to facilitate the uptake and release mechanisms. This, along with additional design strategies, allows the RNA payload to be efficiently delivered to cells for effective, on-target gene editing. The PEG lipid must be carefully selected to balance the need for physical integrity without interfering with the delivery mechanism.
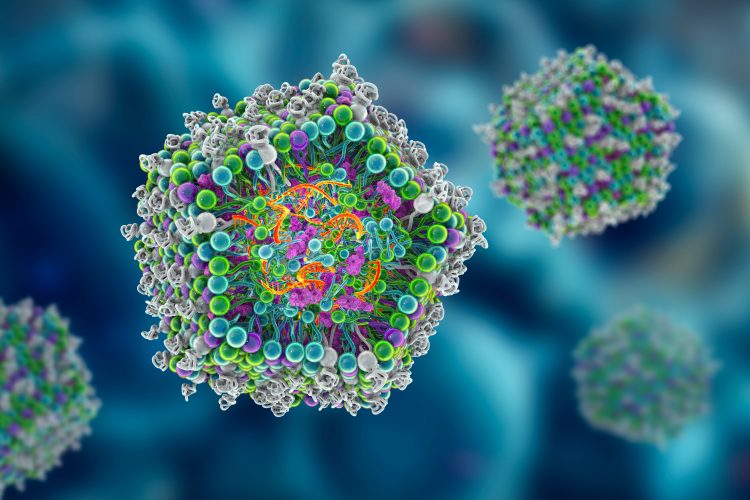

Lipid nanoparticles (LNPs) are tiny, uniform particles made of lipids that encapsulate and deliver nucleic acid drugs such as mRNA, siRNA or CRISPR components into target cells. Image credit: Kateryna Kon / Shutterstock
The evolution of LNP payloads
The first approved clinical usage of LNP was in the delivery of small interfering RNA (siRNA), medicines that result in degradation of target mRNA and consequently reducing the expression of disease-causing genes. Due to their polar nature and moderate size, siRNA needed a stable and non-toxic delivery vehicle that could accommodate the payload, making LNPs an ideal platform. The siRNA-LNP drug Onpattro® was approved by the US Food and Drug Administration (FDA) in 2018 to treat an otherwise fatal disease known as hereditary transthyretin amyloidosis (hATTR), becoming the first approved RNA interference-based drug, as well as the first LNP-formulated nucleic acid drug.
The first approved clinical usage of LNP was in the delivery of small interfering RNA (siRNA).
The development and approval of Onpattro highlighted the potential for LNP to be utilised in the delivery of other nucleic acid therapeutics, some of which were too large for delivery through other modalities, such as AAVs. To date, LNP platforms have been employed across a variety of payload modalities, ranging from the delivery of siRNA for hATTR to mRNA for COVID-19 vaccination to co-delivery of mRNA and guide RNA for CRISPR-Cas9 genome editing. LNPs have repeatedly demonstrated their safety and efficacy over this broad range of applications.
Advantages of LNP
Beyond the versatility of accommodating a variety of payloads with different properties, several qualities make LNPs an advantageous choice for CRISPR delivery over viral systems.
Beyond the versatility of accommodating a variety of payloads with different properties, several qualities make LNPs an advantageous choice for CRISPR delivery over viral systems.
AAV-vector capsids are similar to those of wild-type viruses, with the potential of pre-existing immunity to the viruses due to previous natural exposure. This could impair the delivery and effectiveness of CRISPR therapies. LNPs do not have the same issues.
When comparing immunogenicity, AAV-delivered CRISPR therapies are also limited to a single treatment due to their high immunogenicity, since the activity of subsequent doses would be neutralised by the immune response induced by the first dose. LNPs, however, have a much lower immunogenicity profile, enabling safer administration of CRISPR therapies and making it possible to administer multiple doses. This capability opens the potential of ‘dosing to effect’ where the CRISPR medicine can be re-dosed until the desired therapeutic effect is achieved.
LNP delivery is also transient in its nature of CRISPR expression. Gene therapy through viral vectors typically induces long-term, stable expression of the CRISPR protein, which lessens the potential to mediate unintended changes to the DNA at non-target sites in the genome. In contrast, CRISPR protein expression following mRNA-LNP delivery is high but transient, reducing the likelihood of these unintended, off-target edits.
Additionally, the bioprocesses involved in the manufacturing of AAVs for gene therapy can be time-consuming, difficult to scale up and control, and result in inconsistent performance. ManufacturingAAVs is a complex and lengthy process that may take several weeks, including cell culture growth, transfection, purification and concentration. In contrast, LNP manufacturing processes can generally be completed within one or two days, and potentially only a few hours for personalised applications.
Challenges and risks
As for any delivery method, LNPs have their respective challenges and risks. Systemically administered LNP efficiently deliver their payload to hepatocytes, making them ideal for CRISPR medicines to treat liver-associated diseases. However, biodistribution to cell targets outside the liver can be challenging, limiting the effectiveness of LNP-based CRISPR therapies for diseases that require editing in other cells and organs. Finally, while the immunogenicity for LNP is lower than that of viral vectors, they may still trigger an immune response, which makes it necessary to follow the same strategies to protect and monitor patient safety as with other modalities.
Landmark study: world’s first personalised CRISPR therapy in an infant
In 2025, the Children’s Hospital of Philadelphia and the University of Pennsylvania conducted a groundbreaking single-patient clinical trial for the world’s first-in-human, personalised CRISPR therapy; it was delivered by LNP. The infant, diagnosed with severe carbamoyl-phosphate synthetase 1 deficiency, which has an estimated 50 percent mortality rate, received three escalating doses with no serious adverse events. Incredibly, this therapy was developed and administered to the patient within six months.
In 2025, the Children’s Hospital of Philadelphia and the University of Pennsylvania conducted a groundbreaking single-patient clinical trial for the world’s first-in-human, personalised CRISPR therapy; it was delivered by LNP.
LNPs from Acuitas Therapeutics played a critical role in delivering this therapy, using a formulation containing ionisable lipid ALC-0307™ and PEG-lipid ALC-0159™. The trial presents a new clinical blueprint for rapid-response, personalised gene-editing therapeutics that leverages the availability of a clinically approved delivery platform already in use across multiple clinical indications. Given this successful foundation, further innovations are progressing, such as bedside assembly of therapeutic with personalised RNA guides and mRNA-encoded gene editor to allow more modular and streamlined development and administration.
Such a future would make strategic collaborations crucial, as was the case in this clinical trial. Cross-functional partnerships between Acuitas and payload manufacturers Aldevron and Integrated DNA Technologies enabled exceptionally rapid development and production timelines for the personalised therapy. With each collaborator contributing specialised capabilities and expertise, the components of the treatment were developed and administered safely and efficiently.
Emerging applications and future directions
LNP delivery approaches continue to evolve as researchers seek to expand their capabilities to help patients with a broader range of diseases. One direction is expanding the range of target cells beyond those of liver cells to which LNPs can deliver CRISPR medicines. Designed ankyrin repeat proteins (DARPins), which are antibody mimetic proteins with highly specific and high-affinity target binding, represent a particularly promising approach. Acuitas has used DARPin-conjugated LNP formulations to achieve up to 98 percent binding and approximately 90 percent expression in human CD8⁺ T cells, demonstrating the feasibility of delivering to cells outside the liver.
Another important step is to establish predictable safety profiles of gene therapy medicines delivered with LNP, particularly in the context of repeated dosing. Establishing these profiles safeguards patients by enabling the identification of risks, allowing a therapy to be administered to its greatest effect and helping to expedite the approval of new therapies. Acuitas has conducted research on repeated dosing in non-human primates, showing consistent pharmacokinetics, pharmacodynamics and safety metrics supporting repeat CRISPR administration.
Emerging areas of interest include expanding the application of LNP-based CRISPR medicines from primarily genetic conditions to applications in oncology and cardiovascular disease. Further and equally essential is finding ways to make gene therapies more broadly accessible to patients across a range of socioeconomic groups, as current viral vector-based approaches are prohibitively expensive. Streamlining the development and manufacturing of these products, such as establishing an approved LNP delivery platform for CRISPR therapies, will help to lower costs for drug developers and patients alike.
Work continues to achieve the fullest potential of CRISPR therapies. Delivery platforms such as LNP play a critical role in enhancing and accelerating the development and manufacture of LNP–CRISPR therapeutics for more patients, young and old, across the world.
Meet the authors


Ying K. Tam, Chief Scientific Officer of Acuitas Therapeutics, is a globally recognised expert in nanotechnology and immunology. He directs Acuitas’ scientific strategy and partnerships and has authored more than 100 peer-reviewed studies. He earned his MSc and PhD in developmental and molecular biology from the University of Waterloo and completed a post-doctoral fellowship in cancer immunotherapy at the BC Cancer Agency. He has also held roles in academia, including Assistant Professor at Rush-Presbyterian–St. Luke’s Medical Center in Chicago and Adjunct Professor at the University of British Columbia.


Sean Semple, Vice President of Preclinical Research at Acuitas Therapeutics, has more than 30 years’ experience in the development of lipid nanoparticle therapeutics. As the inventor and pioneer of the ionisable lipid approach to formulate and enable nucleic acids in LNPs, Sean has led numerous first-in-class oligonucleotide–LNP and siRNA–LNP non-clinical programmes into clinical development. He has held positions of increasing responsibility at Arbutus Biopharma, Tekmira Pharmaceuticals and Inex Pharmaceuticals, and is actively engaged with Acuitas’ partners in several gene-editing clinical trials.


Christopher J. Barbosa, Vice President of Technology Development at Acuitas Therapeutics, leads the internal development of the company’s core lipid technology and associated clinical drug product manufacturing technologies. He also oversees the transfer of these technologies to enable Acuitas’ partners to reach the clinic and commercialisation as safely, effectively and rapidly as possible. Chris obtained his PhD in Chemistry and Biotechnology from the University of British Columbia and has held leadership roles in novel drug delivery system R&D at INEX Pharmaceuticals and Angiotech Pharmaceuticals.


Rob Leone, Senior Director of Chemistry, Manufacturing and Controls (CMC) and Process Development (PD) at Acuitas Therapeutics, oversees the development of LNP manufacturing processes to support the clinical translation and commercialisation of nucleic acid therapeutics for partners. Rob earned his BSc in Microbiology and Immunology and an MSc in Biotechnology from the University of British Columbia. He has more than 25 years’ experience in pharmaceutical product development, specialising in novel drug delivery systems, establishing robust and scalable manufacturing processes, and technology transfer.


Paulo Lin, Director of Formulation Development at Acuitas Therapeutics, has nearly 20 years’ experience in the lipid nanoparticle field. He has contributed to more than 60 peer-reviewed publications, including in high-impact journals such as Cell and Nature, and is listed as an inventor on 17 patent families with 110 active applications and 26 granted patents worldwide. Paulo earned his BSc (Hons) and PhD in Biochemistry and Molecular Biology from the University of British Columbia. At Acuitas, he and his team have supported hundreds of programmes, contributing to more than 30 licensed products, including major advances in direct LNP preparation and the optimisation of manufacturing processes.
Related topics
Analysis, Assays, CRISPR, DNA, Drug Delivery, Formulation, Gene Therapy, Genome Editing, Genomics, Immunogenicity, Nanomedicine, Nanoparticles, Nanotechnology, RNAs
Related organisations
Acuitas Therapeutics
Related people
Dr Chris Barbosa (Vice President of Technology Development - Acuitas Therapeutics), Dr Paulo Lin (Director of Formulation Development - Acuitas Therapeutics), Dr Ying K. Tam (Chief Scientific Officer - Acuitas Therapeutics), Rob Leone (Senior Director of CMC and Process Development - Acuitas Therapeutics), Sean Semple (Vice President of Preclinical Research - Acuitas Therapeutics)