New gene therapy restores brain function in SYNGAP1 disorder
Posted: 10 October 2025 | Drug Target Review | No comments yet
Scientists have developed a new gene therapy that reversed symptoms of SYNGAP1-related brain disorders in mice, which could lead to new treatments for this group of neurological conditions.

In a new study, researchers at the Allen Institute have designed a new gene therapy that successfully reversed symptoms of SYNGAP1-related disorders (SRDs) in mice. These disorders are a group of brain conditions that can cause intellectual disability, epilepsy, motor problems and risk-taking behaviours in humans. SRDs usually occur when an individual has only one working copy of the SYNGAP1 gene instead of the usual two.
This is the first time scientists have used an adeno-associated virus (AAV), which carry therapeutic genes into cells, to deliver a working copy of the SYNGAP1 gene into brain cells – which could lead to new ways of treating genetic brain diseases.
“Gene supplementation is providing a functional new copy of a defective gene, a strategy that has great potential for correcting diseases where a gene is completely missing or where a single copy of a gene is lost,” said Dr Boaz Levi, associate investigator at the Allen Institute and senior author of the study. “This provides a clear demonstration that SYNGAP1-related disorders can be treated with a neuron-specific gene supplementation strategy. It’s an important milestone for the field that provides hope for those who suffer from this class of severe neurological diseases.”
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
Dramatic improvements across multiple symptoms
The treated mice showed a near-complete elimination of epileptic brain activity, along with huge reductions in hyperactive and risk-taking behaviours. It also helped restore normal brain wave patterns – a finding that is important due to the fact abnormal rhythms are linked to cognitive issues affecting learning, memory and attention.
The treated mice showed a near-complete elimination of epileptic brain activity, along with huge reductions in hyperactive and risk-taking behaviours.
The study also found that timing played an important role. The researchers treated juvenile mice, similar to the age at which children are typically diagnosed. Despite symptoms already being present, treatment at this stage still led to dramatic improvements – suggesting that gene therapy could remain effective even after disease onset.
This finding gives researchers and families hope that the approach could restore brain function in children with SRDs, not just prevent decline.
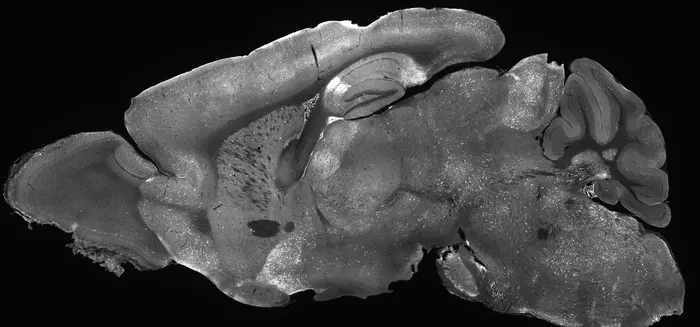

Mouse brain image glowing with experimental gene supplementation therapy highlighting how AAV delivers treatment across the entire brain. Credit: Allen Institute/Andrew Clark
A step toward real treatments for families
Currently, treatment options for SYNGAP1-related disorders are extremely limited. Current available therapies mainly aim to manage symptoms, not address the genetic cause. This study could eventually lead to a future where therapies directly target the root cause of the condition rather than just easing it symptoms.
If future human trials confirm the results seen in mice, the therapy could provide long-awaited relief for families living with these devastating disorders.
Overcoming technical barriers and looking forward
The SYNGAP1 gene is too big to fit into standard AAV delivery systems, so to overcome this, researchers engineered an oversized version of the gene and successfully packed it into the virus. The gene was then successfully delivered into targeted brain cells, where it restored normal function.
While clinical trials in humans are still needed, this study suggests that an effective treatment for SRDs – and the life-altering symptoms caused by them – may finally be possible.
Related topics
Animal Models, Cell Therapy, Central Nervous System (CNS), Drug Discovery Processes, Gene Therapy, Neurosciences, Protein Expression, Sequencing, Therapeutics, Translational Science, Viral vectors
Related conditions
brain conditions, Epilepsy
Related organisations
the Allen institute