How nanocarriers and VLPs are uniting to fight cancer
Posted: 14 October 2025 | Drug Target Review | No comments yet
Innovations in nanomedicine are merging to redefine precision oncology. From virus-like particles to magnetic nanoparticles, integrated delivery systems are showing powerful potential for new, targeted cancer treatments.


Cancer remains one of the leading causes of death worldwide. Traditional therapies, such as chemotherapy and radiotherapy, are often hampered by their lack of specificity, resulting in systemic toxicity and the emergence of drug resistance. However, a new review, explains how nanoparticles offer a promising alternative. Their unique physicochemical properties allow them to traverse biological barriers and they can be engineered for active targeting – either through ligands that bind overexpressed cancer cell receptors or via passive targeting that exploits the Enhanced Permeability and Retention (EPR) effect of tumour vasculature.
Nanocarriers in cancer drug delivery
A wide range of nanocarriers has been developed, each with distinct advantages and limitations:
- Liposomes – spherical phospholipid vesicles that improve drug solubility and pharmacokinetics. These were the first nanocarriers to be tested clinically.
- Solid lipid nanoparticles (SLNs) – offer excellent physical stability and controlled drug release.
- Polymeric nanoparticles (PNPs) – derived from natural or synthetic polymers, they provide versatile options for drug encapsulation and surface functionalisation.
- Dendrimers – highly branched macromolecules capable of displaying multiple surface groups and encapsulating drugs within internal cavities.
- Inorganic nanoparticles – including silica, carbon-based and magnetic nanoparticles, they bring unique features such as high surface area, conductivity and responsiveness to external stimuli like magnetic fields.
Many liposomal and polymeric formulations have already received regulatory approval, demonstrating their translational potential in clinical settings.
Magnetic hyperthermia: combining heat and nanotech
Magnetic hyperthermia represents a minimally invasive approach to cancer therapy. It involves delivering magnetic nanoparticles, such as iron oxide, directly into tumours. When exposed to an alternating magnetic field (AMF), these nanoparticles generate localised heat approximately 42–46 degrees, selectively damaging cancer cells through protein denaturation, DNA disruption, and apoptosis, while sparing healthy tissue.
The true potential of this technique lies in its synergistic applications. Magnetic hyperthermia can sensitise tumours to chemotherapy and radiotherapy and nanoparticles can be co-loaded with drugs for triggered, heat-activated release.
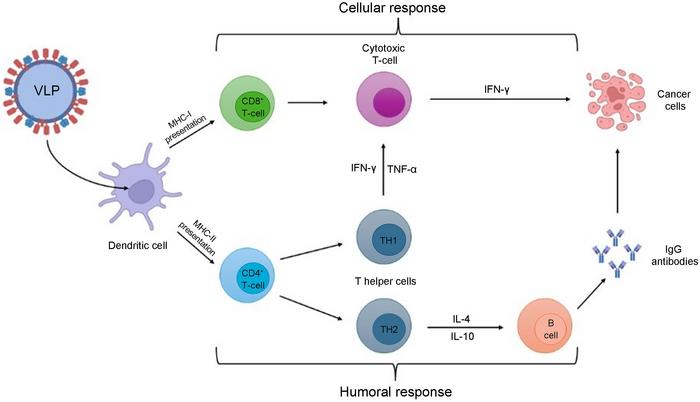

Upon administration, VLPs are uptake by APC, like dendritic cells. VLPs can be presented by MHC-I or MHC-II, which are recognized by CD8+ and CD4+ T cells, respectively. For cellular response, CD8+ cells differentiate in cytotoxic T cells, releasing IFN-γ to exert their cytotoxicity activity in cancer cells. In humoral response, CD4+ cells differentiate into T helper cells (TH1/TH2), TH1 maintains the activity of cytotoxic T cells, and TH2 releases IL-4 and IL-10, inducing B cell activation. Once activated, B cells release IgG antibodies, enabling their antineoplasic effects. APC, antigen-presenting cells; IFN- γ, interferon-gamma; IgG, immunoglobulin G; IL-4/-10, interleukin-4/-10; MHC-I/II, major histocompatibility complex class I/II; TH, T helper cells; TNF-α, tumor necrosis factor-α; VLP, virus-like particles. Credit: Janaina Fernandes
Harnessing viral nanoparticles
Moving beyond synthetic systems, viral nanoparticles (VNPs) and virus-like particles (VLPs) leverage nature’s efficiency.
- VNPs – derived from plant, bacterial or mammalian viruses and may contain genetic material.
- VLPs – a subclass of VNPs, are non-infectious since they lack a viral genome but retain the capsid structure.
Their innate biocompatibility, precise structural organisation and natural tropism make them ideal platforms for drug delivery. They can be produced in expression systems such as yeast, functionalised with targeting ligands and loaded with drugs, genes or imaging agents.
Upon administration, VLPs are taken up by antigen-presenting cells (APCs), such as dendritic cells. These can then present antigens via MHC-I or MHC-II, activating CD8+ cytotoxic T cells and CD4+ T helper cells, which in turn drive B cell activation and antibody production, enabling antineoplastic effects.
Combining strategies for maximum impact
The greatest advances in nanomedicine emerge when these technologies are combined:
- VLPs and magnetic hyperthermia – VLPs can encapsulate chemotherapeutics, such as doxorubicin and be decorated with targeting molecules like folic acid. When paired with magnetic hyperthermia, localised heat triggers drug release directly within tumours, improving both specificity and efficacy.
- Intranasal delivery for brain tumours – The blood-brain barrier (BBB) is a major obstacle for therapy. Intranasal delivery bypasses the BBB by transporting drugs directly to the brain via the olfactory and trigeminal nerves. This route is being explored for delivering oncolytic viruses and VLPs to treat aggressive brain tumours such as glioblastoma.
- Hybrid nanocarriers – To overcome VLP limitations—such as payload capacity and stability—innovative hybrids are being developed. Examples include VLPs conjugated to gold nanoparticles for enhanced photothermal therapy, VLPs coated onto magnetic nanoparticles to improve dispersibility and biomimetic silica nanocages templated from VLPs to increase cellular uptake and biocompatibility.
Conclusions and future directions
The integration of advanced nanocarriers, VLPs and magnetic hyperthermia offers a multi-faceted, precision approach to treating cancer. While synthetic nanoparticles have already developed drug delivery, combining them with biologically inspired carriers and thermo-therapeutic strategies opens new possibilities for targeted, controlled drug release and immune activation.
However, challenges do still remain, particularly in large-scale manufacturing and clinical translation. Continued research is will be crucial to overcome these barriers and transform these promising approaches from experimental concepts into life-saving therapies.
Related topics
Cancer research, Drug Delivery, Nanomedicine, Nanotechnology, Oncology, Polymers, Therapeutics, Translational Science, Viral vectors
Related conditions
Cancer