Brain’s circHomer1 RNA offers insights for future neurological treatments
Posted: 23 October 2025 | Drug Target Review | No comments yet
A circular RNA called circHomer1 has been found to play a vital role in forming and adjusting synapses in developing mouse brains, revealing an overlooked mechanism that helps visual neurons respond to changes in sensory input.

Neuroscientists at MIT have discovered that a circular RNA, circHomer1, helps develop synapses in the brains of mice. The findings demonstrate that circHomer1 is crucial for normal synapse formation and for the brain’s ability to adjust when visual input is temporarily blocked.
Building synapses with circHomer1
“It is used to build synapses, for sure,” said co-senior author Mriganka Sur, Newton Professor at MIT. “When you knock it down, the synapses, at least structurally, are not fully built. Then, after monocular deprivation, when the dendritic spines housing synapses normally should shrink, as should responses from the blocked eye, knocking down circHomer1 prevented that for three days.”
In the study, mice with disrupted circHomer1 showed impaired dendritic spine development and delayed adaptation when one eye was temporarily closed, a procedure called monocular deprivation (MD).
Linear vs circular RNA
The Homer gene produces both linear RNA (Homer1a) and circular RNA (circHomer1). While Homer1a weakens excitatory synapses in response to neuronal activity, the new study shows that circHomer1 plays a distinct and crucial role in shaping synapses during development.
The Homer gene produces both linear RNA (Homer1a) and circular RNA (circHomer1).
The team screened for RNAs affected by MD and found 73 circular RNAs with significant changes. CircHomer1 stood out because its expression rose during the first three days of MD, even as Homer1a levels fell, before declining by day seven. During normal development, circHomer1 also increases at the start of the brain’s ‘critical period,’ when circuits undergo intense remodelling.
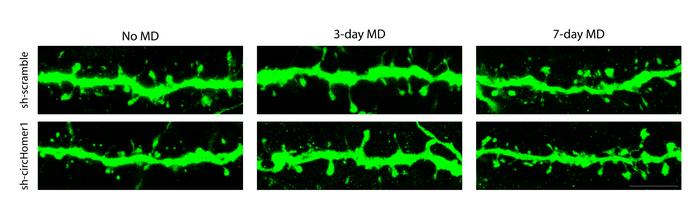

A panel from the paper shows sample neural dendrites under six different conditions: with normal circleHomer1 function (top row) or function knocked out (bottom row), and with no monocular deprivation (left), after 3 days (middle) or 7 days (right). With circleHomer1 disrupted, spines were lessened with no MD. After 3 days of MD circleHomer1 disruption prevented spines from shrinking. After 7 days of MD it made no difference. Credit: Sur Lab/MIT Picower Institute
Effects of knocking out circHomer1
By disabling circHomer1, the researchers observed four conditions: with or without the RNA and with or without MD.
- In non-MD mice – circHomer1 disruption prevented dendritic spine maturation.
- In MD mice with normal circHomer1 – neurons serving the deprived eye showed the expected drop in responsiveness and shrinkage of dendritic spines.
- In MD mice lacking circHomer1 – these changes were delayed for the first three days with reduced removal of AMPA receptor proteins, which normally aids spine shrinkage.
By day seven, however, all effects of circHomer1 loss had vanished, suggesting that other mechanisms may compensate for its absence.
Implications beyond the visual cortex
The study raises questions about how circHomer1 interacts with Homer1a and what mechanisms carry out synaptic adjustments when circHomer1 is missing. While the research focused on the visual cortex, co-author Nikolaos Mellios has been examining circHomer1 in other brain regions, including the prefrontal cortex.
The discovery of circHomer1’s role in synapse development also opens exciting possibilities for future treatments of neurological disorders. By finding a previously overlooked molecular mechanism that shapes how neurons form and adjust connections, the research could guide the development of future therapies aimed at enhancing brain plasticity.
Related topics
Central Nervous System (CNS), Molecular Biology, Neurons, Neurosciences, RNAs
Related conditions
Neurological disorders
Related organisations
MIT
Related people
Mriganka Sur (Newton Professor at MIT)