Cancer immunotherapy: GDF-15’s role in Anti-PD-1 resistance
Posted: 24 August 2023 | Taylor Mixides (Drug Target Review) | No comments yet
In this interview with Christine Schuberth-Wagner, Chief Scientific Officer at CatalYm, we discover research uncovering a central factor contributing to anti-PD-1 resistance in cancer immunotherapy.
The focus of this study is on Growth Differentiation Factor-15 (GDF-15), a cytokine known for its critical role in feto-maternal tolerance, which has now been found to be hijacked by tumours to evade immune attack. We will explore how GDF-15 impacts the tumour microenvironment and hinders the infiltration of T cells into the tumour, as well as the implications of neutralising GDF-15 to reverse its inhibitory effects and sensitise tumours to anti-PD-1 treatment.
What is the central factor identified in the preclinical data that contributes to anti-PD-1 resistance in cancer immunotherapy?
Growth Differentiation Factor-15 (GDF-15) is a cytokine known for its essential function in feto-maternal tolerance, an immunosuppressive mechanism that protects the foetus from the mother’s immune system. Tumours have hijacked GDF-15 and overexpress it to protect themselves from immune attack. The current study is the first to demonstrate a mechanistic link between tumour-produced GDF-15 and the LFA-1/ICAM-1 cell adhesion axis, resulting in impaired infiltration of T cells into the tumour microenvironment. Neutralising GDF-15 with CatalYm’s anti-GDF-15 antibody visugromab was shown to reverse its inhibitory effects and to re-sensitise tumours to anti-PD-1 treatment, achieving commensurate survival benefit of anti-GDF-15-anti-PD-1 combination therapy in vivo.
How does GDF-15 impact the tumour microenvironment and the infiltration of T cells into the tumour?
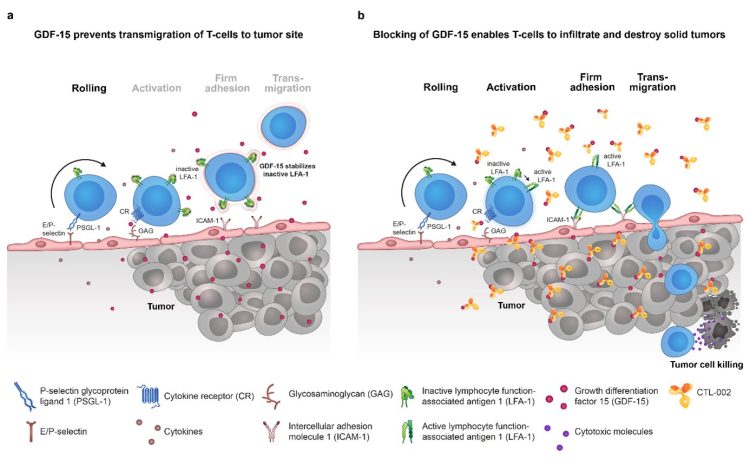
Infiltration of immune cells into tissues follows a cascade of events, namely rolling, activation, adhesion and transmigration, which are mediated by different molecules. Adhesion is mediated via Lymphocyte function-associated antigen (LFA)-1 and Intercellular adhesion molecule (ICAM)-1, which are expressed on the surface of T cells and endothelial cells, respectively. Cancer-derived GDF-15, which is highly overexpressed in more than 50 percent of human solid tumours, blocks T cell recruitment into the tumour microenvironment by inhibiting activation of Lymphocyte function-associated antigen (LFA)-1 which remains unable to interact with Intercellular adhesion molecule (ICAM)-1. By keeping LFA-1 in an inactive conformation, GDF-15 prevents the T cell’s transmigration from the blood vessel to the tumour site (see also graphic below). With this mechanism, GDF-15 enables the tumour to evade being detected and destroyed by immune cells.
What are the implications of neutralising GDF-15 in reversing inhibitory effects and sensitising tumours to anti-PD-1 treatment in the preclinical study?
The study showed that neutralising GDF-15 with CatalYm’s anti-GDF-15 antibody improved T cell infiltration into tumours in mouse models. T-cell infiltration is a prerequisite for responses to checkpoint inhibitors. Combined with a PD-1 inhibitor, it increased tumour clearance and survival with a synergistic effect.
Based on the published data, what is the potential role of GDF-15 as a predictive biomarker for the response to anti-PD-1 therapy and overall survival in melanoma patients?
Microarray analysis of brain metastases from melanoma patients demonstrated a negative correlation between GDF-15 serum levels and T cell infiltration, the latter being a predictive marker for treatment outcome in this indication. These findings were mirrored in the clinical results from an analysis of two metastatic melanoma patient cohorts who received anti-PD-1 treatment: patients with responses and disease control had significantly lower GDF-15 serum levels (measured before treatment start), while none of the patients with elevated GDF-15 levels showed a lasting response. Together with the fact that GDF-15 is overexpressed in more than 50 percent of all solid tumours, these results highlight GDF-15 as a predictive biomarker for failure of immune checkpoint blockade.
Can you explain the significance of the LFA-1/ICAM-1 cell adhesion axis in relation to T cell recruitment into the tumour microenvironment and its relevance to immune checkpoint blockade?
The interaction between LFA-1/ICAM-1 is known to be essential for the extravasation of T cells from the blood vessels into the surrounding tissue.1 Several previous publications demonstrated the importance of this axis for a successful infiltration of T cells into the microenvironment of solid tumours,2 a key factor for the effective destruction of tumour cells and a prerequisite for the success of immune checkpoint blockade therapies, such as anti-PD-(L)1 antibodies.
The results from our study add to these data, demonstrating that tumour-produced GDF-15 blocks LFA-1-dependent T cell recruitment into the tumour microenvironment and, conversely, that blockade of GDF-15 with our anti-GDF-15 antibody visugromab reenables T cell infiltration into tumours in mouse models. Moreover, a combined treatment with a PD-1 inhibitor showed a synergistic effect on tumour clearance and survival, highlighting the synergistic clinical potential of this approach.
How does the preclinical data contribute to the understanding of GDF-15 as a central factor of innate resistance to PD-1 treatment in solid tumour patients?
The findings in this publication reveal a new cancer resistance mechanism of tumour cells that can be either innate or acquired following cancer treatment. “Innate resistance,” in this case, means that cancer cells from the beginning have higher levels of GDF-15 that allow them to circumvent immunotherapies and, therefore, prevent treatment success. Increased GDF-15 levels can, however, also be a reaction to cancer therapy due to tumor cell stress, representing an adaptive mechanism that cancer cells use upon therapeutic pressure to ensure their survival. This mechanism can be triggered by different types of therapy, such as chemotherapy or PD-(L)1 blockage. Our data contribute and expand this body of knowledge by demonstrating that neutralisation of GDF-15 can reverse this immunosuppressive mechanism, with a synergistic effect for the combination of anti-GDF-15 and anti-PD-1 antibodies.
Author bio:
 Christine Schuberth-Wagner
Christine Schuberth-Wagner
Christine Schuberth-Wagner serves as Chief Scientific Officer at CatalYm. She joined the company in November 2018 with more than 10 years of experience in drug discovery and non-clinical development of immunomodulatory drugs in the immuno-oncology space. She has established a track record as a successful leader and entrepreneur in the biotech industry. Prior to joining CatalYm, Christine co-founded Rigontec in 2014 where she led the company’s discovery and non-clinical development activities as Senior Vice President, Research. Her discoveries on the first-in-class RNA-based innate immune receptor agonist RGT100, built the foundation for the initiation of a Phase I clinical trial as well as the successful acquisition of Rigontec by MSD (known as Merck & Co. in North America) in late 2017. Christine holds a PhD in Molecular Biomedicine from the University of Bonn and an MBA from the University of Potsdam.
References
- Nordenfelt P, Elliott HL, Springer TA. Coordinated integrin activation by actin-dependent force during T-cell migration. Nature Communications. 2016 Oct 10;7(1):13119.
- Walling BL, Kim M. LFA-1 in T cell migration and differentiation. Front Immunol 9: 952.
- Piechocka IK, Keary S, Sosa-Costa A, Lau L, Mohan N, Stanisavljevic J, Borgman KJ, Lakadamyali M, Manzo C, Garcia-Parajo MF. Shear forces induce ICAM-1 nanoclustering on endothelial cells that impact on T-cell migration. Biophysical Journal. 2021 Jul 6;120(13):2644-56.
- Springer TA, Dustin ML. Integrin inside-out signaling and the immunological synapse. Current Opinion in Cell Biology. 2012 Feb 1;24(1):107-15.
- Yoong KF, McNab G, Hubscher SG, Adams DH. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. The Journal of Immunology. 1998 Apr 15;160(8):3978-88.
- Hickman A, Koetsier J, Kurtanich T, Nielsen MC, Winn G, Wang Y, Bentebibel SE, Shi L, Punt S, Williams L, Haymaker C. LFA-1 activation enriches tumor-specific T cells in a cold tumor model and synergizes with CTLA-4 blockade. The Journal of Clinical Investigation. 2022 Jul 1;132(13).
- Kantari-Mimoun C, Barrin S, Vimeux L, Haghiri S, Gervais C, Joaquina S, Mittelstaet J, Mockel-Tenbrinck N, Kinkhabwala A, Damotte D, Lupo A. CAR T-cell entry into tumor islets is a two-step process dependent on IFNγ and ICAM-1. Cancer Immunology Research. 2021 Dec 1;9(12):1425-38.
- Harjunpää H, Llort Asens M, Guenther C, Fagerholm SC. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Frontiers in Immunology. 2019 May 22;10:1078. https://doi.org/10.3389/fimmu.2019.01078
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, et. al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014 Nov 27;515(7528):568-71. doi:10.1038/nature13954
- Liu R, Yang F, Yin JY, Liu YZ, Zhang W, Zhou HH. Influence of tumor immune infiltration on immune checkpoint inhibitor therapeutic efficacy: a computational retrospective study. Frontiers in Immunology. 2021 Jun 17;12:685370.
- Li F, Li C, Cai X, Xie Z, Zhou L, Cheng B, Zhong R, Xiong S, Li J, Chen Z, Yu Z, He J, Liang, W. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. eClinicalMedicine. 2021 Nov 1;41. https://doi.org/10.1016/j.eclinm.2021.101134
Related topics
Immunotherapy, Targets
Related conditions
Cancer
Related organisations
Catalym
Related people
Christine Schuberth-Wagner (CataIYm)