How organoids can redefine pre-clinical research
Posted: 22 September 2023 | Dr Han Wei (Beckman Coulter Life Sciences) | No comments yet
Organoids, lab-grown 3D structures that mimic human organs, are redefining preclinical research through bypassing the ethical and practical limitations of animal models. Technological advancements in organoid research, including automation and improved analytical tools, promise to unlock new possibilities by streamlining the application of these 3D structures to enhance drug development and disease modelling.
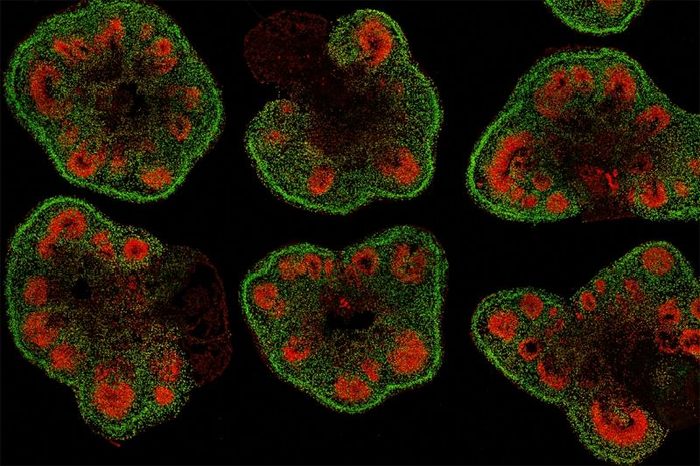
Slices of mini–brain organoids with neural stem cells (red) and cortical neurons (green) [Credit: Hajime Ozaki, Watanabe lab/UCI].
Organoid technologies are becoming an invaluable solution for preclinical research, with the ability to augment the development of personalised medicine, drug discovery and gene therapies.
These 3D cultured structures that are derived from healthy individuals or patients’ cells, such as human pluripotent stem cells (hPSCs)1 or adult stem cells (ADSCs)2 are changing the paradigm of scientific understanding. They have the capacity to differentiate tissue-specific cell types that mimic the architecture and complexity of human organs, from brain,3 to gut,4 providing a platform for preclinical testing of new drugs, high-throughput drug screening and long-term toxicity screening.
The substantial value of organoids is becoming increasingly recognised by supportive government initiatives developing novel drugs, with a growth rate of 22 percent between 2023 and 2030 and a market size predicted to reach over US $6.5 billion by 2030.
Organoid development
The development process of organoids begins with the extraction of adult stem cells or pluripotent stem cells from an organ, followed by their incubation in a specialised in vitro environment containing various growth factors (eg, EGF, FGF, NOG, RSPO1, BMP2) to support the growth, proliferation, and differentiation into specialised cells. This ultimately leads to the formation of a 3D structure that resembles the desired organ.
The incubation time varies from a couple of weeks to a few months, depending on the targeted tissue, and sizes typically range from around 100 micrometres (µm) to a few millimetres (mm) in diameter (5). Comprehensive analytical methods are then employed to evaluate the organoid’s cellular composition (e.g., transcriptomics, scRNA-seq), structure (eg, immunofluorescent staining, bright-field imaging), and functions (eg, ELISA, Luciferase assays).5
Organoids are recognised as New Alternative Methods (NAMs) in drug development. This recognition was codified by the United States Food and Drug Administration (FDA) in 2022 with the signing of the FDA Modernisation Act 2.0 into law.6 As such, in vitro organoid methods can now be accepted either as substitutes for, or in conjunction with, animal studies such as mouse models, which have been extensively used for preclinical validation due to their physiological and genetic similarity to humans.
Mouse model challenges
The use of mouse models has ethical considerations, but additionally, there are inherent drawbacks, including inter-species variability, which can limit the translatability of findings. Mice are often expensive and time-consuming to maintain and breed.7 Apart from bypassing ethical issues, organoids can provide faster and more robust outcomes than animal models, as they are more readily accessible and economical to use. Moreover, their intricate 3D structure and cellular diversity offer a more precise replication of specific structures and functions, making them promising candidates in disease modelling and drug testing.
The ability to develop organoids from patient-specific induced pluripotent stem cells (iPSCs) allows for capturing human diversity in preclinical drug development and opens the door to personalised medicine.
Organoid challenges
Despite the promising developments in organoid cultures, there are several technical challenges. Among these challenges is the complexity of replicating the environment of the human body, which can consequently lead to variations in the structure and function of organoids. This heterogeneity, present within the same structure or between various organoids, could complicate our understanding of specific mechanisms of action when testing novel drugs in vitro.
Maintaining long-term stability is also a hurdle, as organoids frequently exceed the limit for passive diffusion of oxygen, which can lead to the formation of necrotic tissue in the core.8 Furthermore, the absence of immune, mesenchymal, or endothelial cells limits the applicability of organoids for the assessment of certain therapies, such as immuno-oncology treatments or drug metabolism studies where more complex models are needed.9
Using automation to drive the future of organoids
Nevertheless, the field of organoid research is rapidly progressing to address such technological challenges, matching the demands and expectations of preclinical research. Technological advancements include incorporating automation systems to foster the robustness and reproducibility of organoid systems, streamlining drug development stages and high-throughput drug screening. 10,11 Applying more advanced analytical tools and imaging (eg, high-resolution live imaging, mass spectrometry imaging) will aid real-time tracking and analysis of cellular and molecular processes within the organoids.12
There is also a need for further improvement in the composition and functionality of the organoid systems. This includes the development of multi-lineage organoids aiming to represent interactions among various cells within an organ.13 Another direction involves using CRISPR/Cas9 gene-editing technology to generate genetically modified stem cell-derived organoids for investigating the onset, cause, and treatment of human diseases.14 Finally, implementing micro-manufacturing techniques such as 3D printing or laser cutting can help better control the shape and morphology of organoids.8
Finally, implementing micro-manufacturing techniques such as 3D printing or laser cutting can help better control the shape and morphology of organoids.
These technological advancements allow for the creation of more complex organoid systems that offer advantages over standard 2D culture or animal models. One example, still in the research phase involves organoids from a rectal biopsy from two patients, which has indicated a positive response to the ivacaftor via forskolin-induced swelling assay. The derived drug was given to both patients, to which researchers report the patients saw significant improvement.15
As research and workflows evolve, organoids are becoming an integral part of mainstream drug development, personalised medicine, and testing protocols. This can aid in the development of safer, more effective therapeutic strategies.
Author Bio:
 Dr Han Wei
Dr Han Wei
Dr Han Wei is a Market Development Scientist at Beckman Coulter Life Sciences with a focus on building collaborative relationships with external partners. Prior to joining Beckman Coulter Life Sciences, she worked as a Research Associate and Postdoctoral Fellow at Indiana University School of Medicine. She received her Doctor of Philosophy in Biochemistry & Molecular Biology at the Graduate University of Chinese Academy of Sciences, Beijing, China, and a Master of Medicine in Toxicology at The Academy of Military Medical Sciences, Beijing, China. She also received a Bachelor of Medicine in Clinical Medicine (B.M.E.D., – equivalent to M.D.) at Southern Medical University in Guangdong, China.
References
- Vandana JJ, Manrique C, Lacko LA, Chen S. Human pluripotent-stem-cell-derived organoids for drug discovery and evaluation. Cell Stem Cell [Internet]. 2023 May;30(5):571–91. Available from: https://doi.org/10.1016/j.stem.2023.04.011
- Miranda CC, Cabral JMS. Organoids for cell therapy and drug discovery. In: Precision Medicine for Investigators, Practitioners and Providers [Internet]. Elsevier; 2020. p. 461–71. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780128191781000459
- Eichmüller OL, Knoblich JA. Human cerebral organoids — a new tool for clinical neurology research. Nat Rev Neurol [Internet]. 2022 Nov 17;18(11):661–80. Available from: https://www.nature.com/articles/s41582-022-00723-9
- Matsui T, Shinozawa T. Human Organoids for Predictive Toxicology Research and Drug Development. Front Genet. 2021;12(November):1–14.
- Zhao Z, Chen X, Dowbaj AM, Sljukic A, Bratlie K, Lin L, et al. Organoids. Nat Rev Methods Prim [Internet]. 2022 Dec 1;2(1):94. Available from: https://www.nature.com/articles/s43586-022-00174-y
- Co JY, Klein JA, Kang S, Homan KA. Toward Inclusivity in Preclinical Drug Development: A Proposition to Start with Intestinal Organoids. Adv Biol [Internet]. 2023 Mar 18;2200333:2200333. Available from: 10.1002/adbi.202200333
- Sugimoto S, Sato T. Organoid vs In Vivo Mouse Model: Which is Better Research Tool to Understand the Biologic Mechanisms of Intestinal Epithelium? CMGH [Internet]. 2022;13(1):195–7. Available from: https://doi.org/10.1016/j.jcmgh.2021.06.027
- Hofer M, Lutolf MP. Engineering organoids. Nat Rev Mater [Internet]. 2021 Feb 19;6(5):402–20. Available from: http://dx.doi.org/10.1038/s41578-021-00279-y
- Kim J, Koo B-K, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol [Internet]. 2020 Oct 7;21(10):571–84. Available from: http://dx.doi.org/10.1038/s41580-020-0259-3
- Brandenberg N, Hoehnel S, Kuttler F, Homicsko K, Ceroni C, Ringel T, et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat Biomed Eng [Internet]. 2020 Jun 8;4(9):863–74. Available from: https://www.nature.com/articles/s41551-020-0565-2
- Louey A, Hernández D, Pébay A, Daniszewski M. Automation of Organoid Cultures: Current Protocols and Applications. SLAS Discov [Internet]. 2021;26(9):1138–47. Available from: https://doi.org/10.1177/24725552211024547
- Beghin A, Grenci G, Sahni G, Guo S, Rajendiran H, Delaire T, et al. Automated high-speed 3D imaging of organoid cultures with multi-scale phenotypic quantification. Nat Methods [Internet]. 2022 Jul 13;19(7):881–92. Available from: https://www.nature.com/articles/s41592-022-01508-0
- Tang X-Y, Wu S, Wang D, Chu C, Hong Y, Tao M, et al. Human organoids in basic research and clinical applications. Signal Transduct Target Ther [Internet]. 2022 May 24;7(1):168. Available from: https://www.nature.com/articles/s41392-022-01024-9
- Geurts MH, Clevers H. CRISPR engineering in organoids for gene repair and disease modelling. Nat Rev Bioeng [Internet]. 2023 Jan 19;1(1):32–45. Available from: https://www.nature.com/articles/s44222-022-00013-5
- Bartfeld S, Clevers H. 2017. Stem cell-derived organoids and their application for medical research and patient treatment. J. Mol. Med. 95: 729–38
Related topics
3D printing, Disease Research, Drug Discovery, Organoids
Related organisations
Beckman Coulter Life Sciences
Related people
Dr Han Wei (Beckman Coulter Life Sciences)







