Streamlining the path from lab to market with 3D bioprinting
Posted: 18 March 2024 | Dr Vidmantas Šakalys (Vital3D), Ellen Capon (Drug Target Review) | No comments yet
In this Q&A, Dr Vidmantas Šakalys, CEO of Vital3D, provides insights into the potential of 3D bioprinting to revolutionise the traditional drug development process, facilitating more accurate models and limiting the use of animal testing.
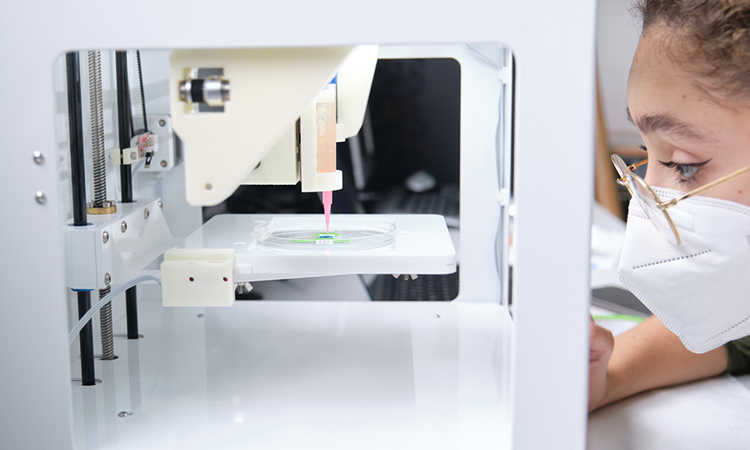

How do recent advancements in bioprinting technology promise to revolutionise the traditional drug development process, in terms of cost and timeline?
Recent advancements in bioprinting technology are setting the stage for a major transformation in the drug development process, primarily through cost reduction and accelerated timelines. Traditional methods, which are often lengthy and expensive, can benefit from bioprinting’s ability to create complex tissue and organ models. These models can be used for more efficient and effective drug testing early in the development process, potentially reducing the need for animal testing and later-stage human trials that are both costly and time-consuming. By enabling a more direct assessment of drug interactions with human tissue, bioprinting can streamline the path from lab to market.
How can 3D bioprinting facilitate drug discovery by providing more accurate models for testing drug effectiveness and safety, compared to conventional methods?
3D bioprinting offers a significant advancement in drug discovery by creating models that closely mimic the intricate structure and functionality of human organs and tissues. These models provide a more accurate environment for testing drug effectiveness and safety than traditional 2D cell cultures or animal models. By replicating the human physiological conditions more closely, bioprinted tissues can reveal the potential effects and side effects of drugs with greater precision, leading to more reliable data on drug efficacy and safety before proceeding to human trials.
What are some of the key challenges that researchers face in utilising 3D bioprinting for drug discovery and regenerative medicine, and what strategies can be employed to address these challenges?
The key challenges in utilising 3D bioprinting for drug discovery and regenerative medicine include replicating the complex biology of human tissues, ensuring vascularisation for tissue survival, and overcoming the potential for immune rejection. Additionally, the technology faces hurdles in the standardisation of tissue sourcing, processing techniques, and the creation of defined tissue engineering matrices. Strategies to address these challenges include advancing the technology to better replicate tissue complexity and physiological conditions, developing standardised protocols for tissue engineering, and enhancing the biocompatibility and functionality of bio-printed tissues through ongoing research and innovation.
What role does 3D bioprinting have in mitigating ethical concerns and scientific limitations associated with animal testing?
3D bioprinting plays a pivotal role in addressing both ethical concerns and scientific limitations of animal testing. By providing human-like models for drug testing and disease modelling, bioprinting reduces the reliance on animal models, aligning with ethical considerations and improving the relevance of experimental results to human health. This technology offers a promising alternative that can replicate human physiology more accurately than animal models, potentially leading to more ethically sound and scientifically valid research outcomes.
Can you elaborate on specific instances where 3D bioprinting technology has demonstrated significant impact within the healthcare industry, and where it offers the potential for future breakthroughs?
- Tissue and organ models. They help scientists to better understand organ function and disease mechanisms.
- Miniature organs, often referred to as organoids. These are used for drug testing, disease modelling, and personalised medicine.
- Drug delivery devices. 3D printing allows the creation of personalised drug delivery devices, such as tablets with controlled-release profiles or complex geometries.
About the author


Vidmantas Šakalys is a top-level business manager with broad working knowledge in technology innovations and ITT management. He has practical experience of over 20 years in ITT management roles and over 10 years in photonics innovation management.
He founded and led laser research start-up Femtika.
Related topics
3D printing, Animal Models, Clinical Trials, Drug Development, Drug Discovery, Organoids, Regenerative Medicine
Related organisations
Vital3D