Why radiopharmaceuticals are gaining ground in the fight against cancer
Posted: 6 June 2025 | Drug Target Review | No comments yet
Radiopharmaceuticals represent a rapidly advancing field in oncology, using radioactive compounds to both detect and treat cancer at the molecular level. This article explores how targeted radiation is improving patient outcomes while reducing systemic toxicity.

In recent years, the field of nuclear medicine has undergone a quiet revolution, particularly in its application to oncology. At the forefront of this transformation is Dr Ebrahim Delpassand, a nuclear medicine physician and the driving force behind RadioMedix (RMX), a radiopharmaceutical company focused on developing targeted diagnostics and therapies. In an interview with our team, Delpassand shared his perspective on how targeted radiopharmaceuticals are reshaping cancer treatment and what lies ahead for this evolving field.
Innovation and patient care
Delpassand is not only the Founder, CEO and Chairman of RadioMedix, but also serves as Chairman and Medical Director of Excel Diagnostics, a sister organisation specialising in clinical implementation. “I’ve dedicated my career to advancing the field of nuclear medicine for both diagnostic imaging and targeted therapies,” he explains.
I’ve dedicated my career to advancing the field of nuclear medicine for both diagnostic imaging and targeted therapies.
This dual role enables him to bridge research and clinical practice, ensuring scientific innovation is directly informed by patient care. “As a CEO of RadioMedix, I seek to find innovative targeted radiopharmaceutical solutions to rid the body of cancer cells without affecting healthy cells,” he says, distinguishing this targeted approach from systemic treatments like chemotherapy or external beam radiotherapy. At RadioMedix, he helps drive strategy, identifies promising candidates and isotopes, and oversees the development of both diagnostic and therapeutic agents – often in the form of theranostic pairs.
Delpassand’s clinical experience plays a key role in shaping the research pipeline. “Protocol development can be a complex and meticulous process,” he explains, “as it’s crucial to ensure that trial candidates meet the necessary inclusion criteria.” Prior treatments and patient histories often introduce variables that can affect outcomes, making thoughtful trial design essential for generating reliable data.
Why targeted radiopharmaceuticals?
The founding of RadioMedix was born from a recognition of unmet needs in cancer treatment. “The impetus to launching RMX was to address unmet needs in the treatment of various cancers, including neuroendocrine tumours (NETs), prostate, brain, pancreatic and ovarian cancers,” says Delpassand. The promise of delivering tiny, precise doses of radioactive material directly to cancerous tissue marked a significant step away from the toxic side effects often associated with traditional therapies.
He references early work from Erasmus University in the 2000s as a turning point, where researchers were targeting NETs using lutetium-177 dotatate (Lu-177). “RMX initiated the first IND for Lu-177 dotatate in the US for NETs at Excel Diagnostic Oncology Center, where we treated our first patient in August 2010,” he recalls. The results were promising: “we viewed a complete pivot in therapy… better outcomes and the patient could receive treatment in the US instead of travelling to Europe.”
This clinical success reinforced their focus on radioligand therapy (RLT), which uses radioactive isotopes linked to molecules that specifically target cancer cells.


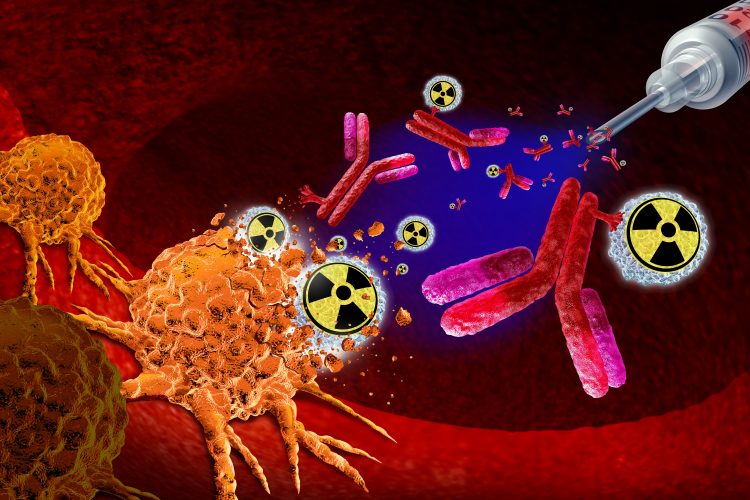
At the heart of radiopharmaceutical therapy is the ability to cause precise DNA damage within cancer cells, disrupting their ability to grow and divide. Image credit: Crystal Eye Media / Shutterstock
The role of SPICA and manufacturing capabilities
Drug development in the radiopharmaceutical space is particularly resource intensive. Beyond the research and clinical testing lies a significant manufacturing hurdle: the ability to produce these specialised drugs in a controlled, scalable environment. This is where the SPICA Center comes in.
Centres dedicated to manufacturing radiopharmaceuticals are one of the unmet needs of our growing space.
“Centres dedicated to manufacturing radiopharmaceuticals are one of the unmet needs of our growing space,” says Delpassand. “The SPICA Center was built to address this gap.” Operating under current good manufacturing practices (cGMP), the facility allows RMX to produce investigational drugs for clinical trials, while preparing for potential commercial scale-up.
GMP compliance is non-negotiable in this field. “RMX addresses this vital task by strict adherence to GMP guidelines, which requires constant training, control of environmental factors during manufacturing, calibration of equipment and strong QA oversight,” he says. These measures ensure both the efficacy and safety of the final product.
End-to-end development
Unlike many organisations in the pharmaceutical sector, RadioMedix maintains an integrated model from pre-clinical development through to commercial manufacturing. “Having the infrastructure, know-how and expert manpower has allowed RMX to play in the entire value chain,” Delpassand explains.
The integrated model is strengthened by close collaboration with Excel Nuclear Oncology Center, allowing faster translation of research into clinical practice. Clinicians contribute directly to the research process, ensuring the work remains aligned with real patient needs.
Shared goals in cancer drug discovery
For all its internal capabilities, RMX does not operate in isolation. “Partnership with other scientists, academic or in industry, and companies, private or public, has been one of our main pillars of success,” says Delpassand. He stresses the importance of recognising organisational strengths and weaknesses: “No single company is fully expert in all aspects of drug development.”
This philosophy guides RMX’s collaborative efforts, whether the partner is focused on discovery, clinical development or commercialisation. “We believe in partnership when it can expedite patient access to innovative drugs,” he adds, noting that sharing expertise – and indeed, a share of success – is vital to accelerating medical progress.
Trends and breakthroughs on the horizon
So, what does the future hold for targeted radiopharmaceuticals? Delpassand sees enormous potential in several difficult-to-treat cancers. “I predict that within the next five to seven years, we will see breakthroughs in the diagnosis and treatment of many hard-to-diagnose cancers, and treat oncological conditions such as brain, pancreatic, colorectal, ovarian and triple-negative breast cancers,” he says.
I predict that within the next five to seven years, we will see breakthroughs in the diagnosis and treatment of many hard-to-diagnose cancers.
Another area of promise is the convergence of radiopharmaceuticals and antibody-drug conjugate (ADC) technology. “This requires collaboration between scientists and companies in nuclear medicine and the ADC space,” he notes.
Manufacturing also remains a critical focus, especially when it comes to alpha emitters – radioisotopes capable of delivering high-energy particles to tumours with minimal damage to surrounding tissues. “Another emerging trend is manufacturing of exotic diagnostic and therapeutic, specifically alpha emitters, radioisotopes,” he explains, suggesting that RMX is already preparing to lead in this complex area.
Conclusion
Delpassand’s work demonstrates the practical application of radiopharmaceuticals in oncology, focusing on precise tumour targeting with minimal impact on healthy tissue. His combined clinical and research experience supports a development model that integrates discovery, trial design and manufacturing to advance treatments for cancers that are difficult to diagnose or treat.


Dr Ebrahim S. Delpassand is a board-certified nuclear medicine physician with extensive experience in Targeted Radionuclide Therapy and Nuclear oncology. He served as Deputy Chair and Chief of Clinical Nuclear Medicine at MD Anderson Cancer Center and founded Excel Diagnostics & Nuclear Oncology Center in 2003. In 2006, he founded RadioMedix, a clinical stage biotechnology company, based in Houston, Texas. Delpassand is the PI and Sponsor of several current INDs and an Adjunct Professor at the Department of Radiation Oncology at University of Texas and Nuclear Medicine at Baylor College of Medicine in Houston. He has authored more than 90 peer reviewed medical manuscripts and presented topics in targeted radionuclide therapies, and PET imaging in multiple national and international congresses.
Related topics
Biopharmaceuticals, Drug Development, Oncology, Personalised Medicine, Radiotherapy, Therapeutics, Translational Science
Related conditions
Cancer
Related organisations
RadioMedix (RMX)
Related people
Dr Ebrahim S. Delpassand (Founder of RadioMedix)