First captured image of antigen-bound T-cell receptor at near-atomic resolution
Posted: 19 August 2022 | Ria Kakkad (Drug Target Review) | No comments yet
Using cryo-electron microscopy, researchers have captured the structure of a membrane-bound T-cell receptor complex with bound antigen.

A team of researchers from Goethe University Frankfurt, the Max Planck Institute of Biophysics, both Germany and the University of Oxford, UK have successfully visualised the structure of a membrane-bound T-cell receptor (TCR) complex with bound antigen for the first time. A comparison of the antigen-bound structure captured using cryo-electron microscopy (cryo-EM) with that of a receptor without antigen provides the first clues to the activation mechanism. The ground-breaking results were recently published in Cell.
For the structural analysis, the researchers chose a TCR used in immunotherapy to treat melanoma and which had been optimised for this purpose in several steps in such a way that it binds its antigen as tightly as possible. A particular challenge on the way to structure determination was to isolate the whole antigen receptor assembly consisting of eleven different subunits from the cell membrane.
Once they had successfully achieved this, the researchers used a strategy to obtain those receptors out of the preparation that had survived the process and were still functional: due to the strong interaction between the receptor complex and the antigen, they were able to find one of the most medically important immune receptor complexes. The subsequent images collected at the cryo-EM delivered ground-breaking insights into how the TCR works.
“On the basis of our structural analysis, we were able to show how the TCR assembles and recognises antigens and hypothesise how signal transduction is triggered after antigen binding,” explained Robert Tampé, one of the lead researchers from the study.
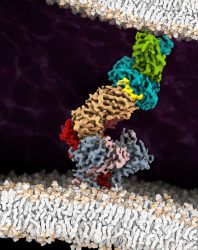

The cryo-EM structure of the fully assembled T-cell receptor (TCR) complex with a tumour-associated peptide/MHC ligand provides important insights into the biology of TCR signalling. These insights into the nature of TCR assembly and the unusual cell membrane architecture reveal the basis of antigen recognition and receptor signalling
[Credit: Robert Tampé, Goethe University Frankfurt].
The scientists were surprised to find that there is evidently no significant change in the receptor’s spatial structure after antigen binding, as this was practically the same both with and without an antigen.
The remaining question is how antigen binding could instead lead to T-cell activation. The co-receptor CD8 is known to approach the TCR after antigen binding and to stimulate the transfer of phosphate groups to its intracellular part. The researchers assume that this leads to the formation of structures which exclude enzymes that split phosphatases. If these phosphatases are missing, the phosphate groups remain stable at the TCR and can trigger the next step of the signalling cascade.
“Our structure is a blueprint for future studies on T-cell activation,” Tampé concluded. “In addition, it is an important stimulus for employing the TCR in a therapeutic context for treating infections, cancer, and autoimmune diseases.”
Related topics
Bioinformatics, Microscopy
Related organisations
Goethe University Frankfurt, Max Planck Institute of Biophysics, The University of Oxford
Related people
Robert Tampé