Researchers establish the first human respiratory organoid culture system
Posted: 2 May 2023 | Izzy Wood (Drug Target Review) | No comments yet
A team from the University of Hong Kong has developed the first human respiratory organoid culture system, using it to unveil a novel mechanism for the high transmissibility of the SARS-CoV-2 Omicron variant.

Researchers from the State Key Laboratory for Emerging Infectious Diseases and the Department of Microbiology at the University of Hong Kong have developed the first human respiratory organoid culture system, and have used it to identify a novel mechanism for the high transmissibility of the SARS-CoV-2 Omicron variant.
The team, led by Dr Zhou Jie and Professor Yuen Kwok-yung, collaborated with Professor Hans Clevers from the Netherlands’ Hubrecht Institute, and Professor Jiang Shibo from Fudan University in China, to investigate the infection and replication capacity of the Omicron BA.5 variant in human respiratory organoids.
The team established the first organoid culture system of the human respiratory epithelium directly from primary lung tissues and nasal cells. These respiratory organoids were stably and consecutively expanded over half to one year, and scientists induced maturation in long-term expandable organoids, generating nasal, airway, and alveolar organoids that contain all major epithelial populations in the human respiratory tract. The organoid culture system enables scientists to reconstruct and expand the entire human respiratory epithelium in culture dishes, providing an optimal tool for studying respiratory biology and diseases, including COVID-19.
The researchers then investigated the infection and replication of the ancestral strain of SARS-CoV-2 and two Omicron subvariants, B.1.1.529 and BA.5, in the airway and nasal organoids. They found that BA.5 exhibited dramatically increased infectivity and replicative capacity compared to B.1.1.529 and the ancestral strain WT in human nasal and airway organoids. BA.5 robustly replicated in human nasal and airway organoids to a comparable level to an influenza virus, pandemic H1N1, and the researchers observed prominent syncytium formation in BA.5-infected nasal and airway organoids. Overall, the higher entry efficiency and fusogenic activity of BA.5 enabled viral spread through syncytium formation in the human nasal and airway epithelial cells, leading to dramatically enhanced infectivity and transmissibility.
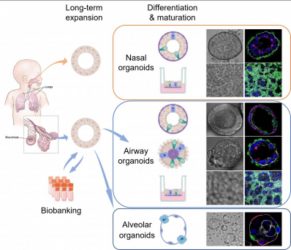

The research team induced maturation in long-term expandable organoids and generated nasal, airway and alveolar organoids that contain all major epithelial cell populations in the human respiratory tract (CREDIT: The University of Hong Kong)
Based on their findings, the team commented that “BA.5 has evolved to gain higher fitness in the human respiratory cells due to the enhanced entry efficiency and fusogenic activity”, which provides scientific evidence for the implementation of public health policies. The research team’s respiratory organoids established are robust and universal tools for studying respiratory biology and diseases.
The emergence of SARS-CoV-2 new variants has caused multiple waves worldwide. Omicron was first detected in South Africa in November 2021 and spread rapidly across the globe. The high transmissibility of SARS-CoV-2 Omicron variants was largely ascribed to immune escape, which led to significantly increased risk of reinfection or breakthrough infection in vaccinated populations. However, the researchers’ study reveals a novel mechanism for the high transmissibility of the BA.5 variant, providing new insights into how the virus is adapting to gain higher fitness in the human respiratory system. The research team’s findings could contribute to future vaccine development and the creation of effective public health policies to limit the spread of SARS-CoV-2.
Related conditions
SARS-CoV-2 Omicron variant.
Related organisations
Fudan University, State Key Laboratory for Emerging Infectious Diseases, the Department of Microbiology at the University of Hong Kong, the Netherlands’ Hubrecht Institute
Related people
Dr Zhou Jie, Professor Hans Clevers, Professor Jiang Shibo, Professor Yuen Kwok-yung