Tiny models, powerful insights: how organoids are driving precision oncology
Posted: 30 September 2025 | Drug Target Review | No comments yet
A new review has highlighted how three-dimensional organoid models are transforming cancer research by replicating the complexity of human tumours – bringing precision oncology closer to the clinic.

Organoid models are reshaping cancer research by replicating the intricate structures and diversity of human tumours. Unlike conventional flat cell cultures or animal models, organoids capture the true complexity of tumour growth, drug resistance and immune responses.
A team from Peking University People’s Hospital has published a new review in Cancer Biology & Medicine. Their work outlines how organoid models are revolutionising cancer research by providing realistic, patient-derived systems to test therapies and accelerate vaccine development.
The review charts scientific progress and highlights the growing role of organoids in shaping the future of precision medicine. Cultivated from patient tumour tissue or stem cells, organoids faithfully reproduce the genetic mutations and microenvironments of the original tumours.
Automation now plays a central role in discovery. From self-driving laboratories to real-time bioprocessing
This report explores how data-driven systems improve reproducibility, speed decisions and make scale achievable across research and development.
Inside the report:
- Advance discovery through miniaturised, high-throughput and animal-free systems
- Integrate AI, robotics and analytics to speed decision-making
- Streamline cell therapy and bioprocess QC for scale and compliance
- And more!
This report unlocks perspectives that show how automation is changing the scale and quality of discovery. The result is faster insight, stronger data and better science – access your free copy today
When combined with technologies such as microfluidics, single-cell sequencing and proteomics, these mini-tumours provide a living laboratory to test drugs, study tumour evolution and even design personalised cancer vaccines.
Limitations of traditional models
For decades, cancer research has relied on simplified models that fall short of replicating the true nature of human tumours. Flat cell cultures often lose genetic integrity and lack the surrounding microenvironment, while animal models are costly, slow and biologically different. These limitations have left a critical gap between laboratory results and clinical success – contributing to high drug failure rates and limited progress in personalised care.
Preserving heterogeneity to predict clinical outcomes
Their strength lies in preserving heterogeneity: in colorectal and gastric cancers, organoid drug-response testing has closely mirrored clinical outcomes. Beyond chemotherapy, organoid co-cultures with immune cells provide a breakthrough platform for studying checkpoint inhibitors and CAR-T therapies – directly linking lab findings with patient survival outcomes.
Technological advances are amplifying these possibilities too. Microfluidic ‘organoid-on-a-chip’ systems mimic dynamic processes like metastasis, while proteomics and single-cell sequencing map hidden signalling pathways and clonal diversity. Together, these tools provide unparalleled insight into tumour biology.
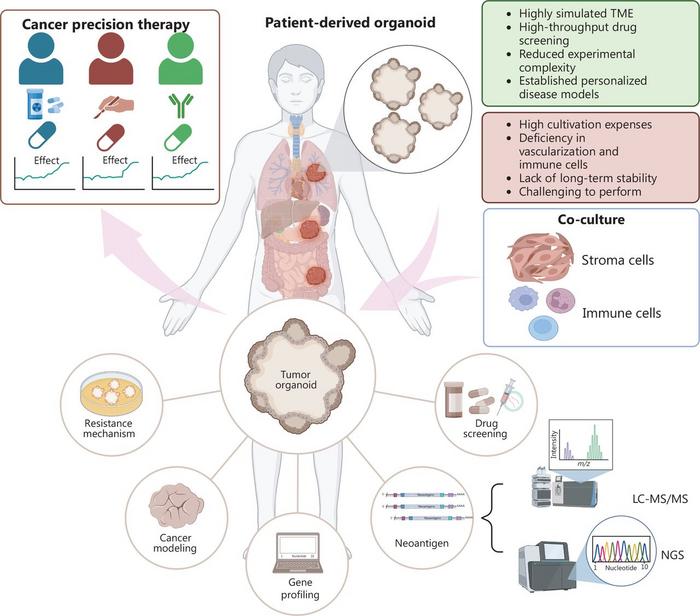

This figure illustrates the central role of tumour organoids in patient-specific cancer research. Patient-derived organoids (PDOs) established from gastrointestinal, pulmonary, breast and other tumour types simulate key aspects of the tumour microenvironment (TME), enabling applications in drug screening, neoantigen identification, resistance mechanism analysis and cancer progression modelling.
Organoids and personalised vaccines
Importantly, organoids are not confined to treatment validation – they also enable antigen screening and vaccine development by preserving tumour-specific features and simulating immune responses in vitro. By merging patient-derived biology with high-throughput and high-resolution technologies, organoids offer researchers the means to move from general cancer models toward individualised treatment strategies.
Importantly, organoids are not confined to treatment validation – they also enable antigen screening and vaccine development by preserving tumour-specific features and simulating immune responses in vitro.
“Organoids have transformed the way we approach cancer research,” said Dr Kezhong Chen, senior author of the review. “They allow us to study tumours as living ecosystems, capturing both genetic complexity and immune dynamics. This means we can test therapies in conditions far closer to reality and predict how individual patients might respond. The potential is immense – not only for refining today’s treatments but also for developing tomorrow’s personalised cancer vaccines. Organoids bridge the gap between the lab and the clinic in ways we could only imagine a decade ago.”
From bench to bedside
Clinicians can now use organoids to guide therapy choices and reduce exposure to ineffective drugs, while researchers gain a platform to explore drug resistance and identify biomarkers with precision. Pharmaceutical pipelines may also become faster and less costly as organoids reduce reliance on animal testing and streamline early-stage trials.
In vaccine development, organoids can personalise immune strategies by predicting patient-specific responses. While challenges remain in culture standardisation and long-term stability, organoids promise to accelerate the transition toward precision oncology and provide cancer patients with more effective, tailored treatment options.
Related topics
Cancer research, Drug Discovery, Drug Discovery Processes, Immuno-oncology, Immunotherapy, Next-Generation Sequencing (NGS), Oncology, Organoids, Precision Medicine, Sequencing, Translational Science, Vaccine development
Related conditions
Cancer
Related organisations
Peking University People's Hospital
Related people
Dr Kezhong Chen (Peking University People's Hospital)