Existing nerve pain drugs may help stop bone cancer spread
Posted: 31 October 2025 | Drug Target Review | No comments yet
Scientists have discovered that two existing pain medications – bupivacaine and rimegepant – may not only relieve the severe pain caused by osteosarcoma but also slow the disease’s growth.

Two drugs currently prescribed for nerve pain and migraines may be able to be repurposed as new treatments for a rare and aggressive bone cancer, according to a new study from researchers at Johns Hopkins Medicine. They found that bupivacaine and rimegepant not only relieve tumour-associated pain in laboratory mice with osteosarcoma-like tumours but also appear to slow cancer growth too.
Repurposing existing drugs
“Our findings suggest that these two medications – already approved by the US Food and Drug Administration (FDA) for relieving nerve pain and migraines – might one day be repurposed as anti-tumour therapies,” said study lead author Dr Sowmya Ramesh, a postdoctoral researcher in pathology at the Johns Hopkins University School of Medicine.
Our findings suggest that these two medications – already approved by the US Food and Drug Administration (FDA) for relieving nerve pain and migraines – might one day be repurposed as anti-tumour therapies.
“That’s because our study shows that these drugs affect three proteins: calcitonin gene-related peptide (CGRP), tryptomyosin receptor kinase-A (TrkA) and nerve growth factor (NGF), inhibiting their neuron-to-tumour signalling and keeping them from stimulating innervation and angiogenesis in osteosarcomas.”
Biomarkers are redefining how precision therapies are discovered, validated and delivered.
This exclusive expert-led report reveals how leading teams are using biomarker science to drive faster insights, cleaner data and more targeted treatments – from discovery to diagnostics.
Inside the report:
- How leading organisations are reshaping strategy with biomarker-led approaches
- Better tools for real-time decision-making – turning complex data into faster insights
- Global standardisation and assay sensitivity – what it takes to scale across networks
Discover how biomarker science is addressing the biggest hurdles in drug discovery, translational research and precision medicine – access your free copy today
Later in the study, the researchers observed that using these two drugs on mice with osteosarcoma-like tumours inhibited nerve and blood vessel formation in the tumours and stopped the cancer’s growth and spread. The long-term aim is that this may develop into a treatment for human osteosarcomas.
Turning previous research on its head
Ironically, the new findings represent a reversal of earlier work from the same laboratory. They demonstrated that NGF-TrkA signalling by peripheral neurons increased the sprouting of sensory nerves and blood vessels within mice. This is crucial in fracture repair, so their goal was to increase the amount of those proteins.
“Now, instead, we want to curtail this peripheral nerve signalling to prevent innervation and angiogenesis in osteosarcomas and in turn, reduce or stop the tumour’s growth and spread,” said Professor Aaron James, the study’s senior author and leader of the James Laboratory at Johns Hopkins.
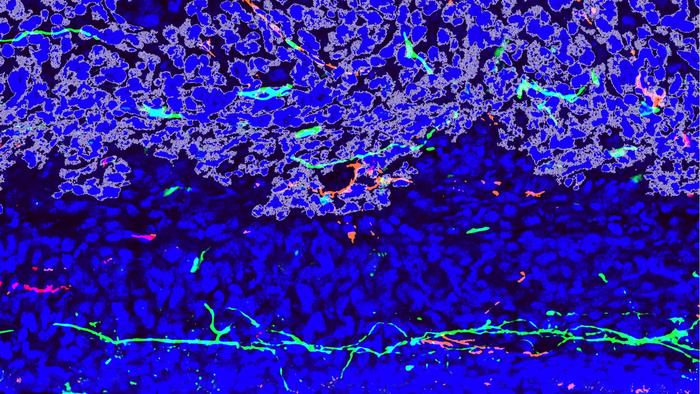

A confocal microscopy image of mouse bone cancer, or murine osteosarcoma (outlined in gray), invading normal bone (blue). Peripheral nerves (green) and blood vessels (red) sprout around the tumor, forming a supportive environment for its growth and spread. Credit: Sowmya Ramesh, Johns Hopkins Medicine.
Mouse model results
The researchers first explored how sensory neurons influence osteosarcoma progression by using genetically modified mice in which TrkA signalling activity was inhibited.
“We found that the mice with inhibited NGF-TrkA signalling had markedly less nerve growth,” said Dr Ramesh. “Additionally, a large number of the TrkA-inhibited mice showed slower overall tumour growth and spread and prolonged survival.”
When the team examined tissue samples from human osteosarcomas, they found the same NGF-TrkA binding effects increased nerve and blood vessel growth.
The researchers first explored how sensory neurons influence osteosarcoma progression by using genetically modified mice in which TrkA signalling activity was inhibited.
“This suggests that in human osteosarcomas, what was seen in the mouse tumours – NGF-TrkA signalling resulting in increased innervation and angiogenesis – also leads to tumour growth and spread, and sarcoma-induced nerve pain,” said Professor James.
Further analysis of dorsal root ganglion (DRG) neurons – the nerves along the spinal cord that transmit signals from the body to the brain – demonstrated that patients who experienced tumour-related pain showed both CGRP activity and inflammation in their DRG neurons.
Having established the role of CGRP-induced NGF-TrkA signalling in both tumour growth and pain, the researchers tested whether blocking CGRP could tackle both problems.
“We found in our mouse osteosarcoma model that both FDA-approved drugs, bupivacaine and rimegepant, reduced the amount of tumour-associated innervation and angiogenesis seen,” said Dr Ramesh.
Future studies
With evidence that peripheral sensory nerves are closely linked to osteosarcoma growth and spread, the team now plan to study the mechanisms in more depth.
They aim to define exactly how neurons respond to tumour signals – and how those responses might be manipulated to halt cancer progression.
These findings could allow for new drug discovery strategies that target nerve–tumour communication, potentially leading to treatments that both relieve pain and suppress the cancer at its source.
Related topics
Animal Models, Cancer research, Drug Discovery, Drug Repurposing, Neurons, Oncology, Translational Science
Related conditions
Cancer, osteosarcoma
Related organisations
Johns Hopkins Medicine








