Targeting EZH2 may prevent triple-negative breast cancer spread
Posted: 7 October 2025 | Drug Target Review | No comments yet
A new study has revealed that the enzyme EZH2 triggers abnormal cell division that fuels metastasis, and blocking this enzyme with existing drugs could restore normal cell behaviour to stop cancer from spreading.

Triple negative breast cancer (TNBC) is one of the most aggressive and difficult forms of breast cancer to treat. A new study led by Weill Cornell Medicine has discovered a surprising potential strategy to prevent its spread. Researchers identified an enzyme, EZH2, that drives TNBC cells to divide abnormally, enabling them to metastasise to distant organs. Preclinical experiments showed that drugs blocking EZH2 could restore normal cell division and prevent the spread of TNBC cells.
“Metastasis is the main reason patients with triple negative breast cancer face poor survival odds,” said senior author Dr Vivek Mittal, Ford-Isom Research Professor of Cardiothoracic Surgery and member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine. “Our study suggests a new therapeutic approach to block metastasis before it starts and help patients overcome this deadly cancer.”
The findings challenge the idea that cancer treatments should exacerbate cell division errors in tumour cells to induce cell death. Normally, when cells divide, chromosomes duplicate and split evenly into two daughter cells. In many cancer cells, this process goes awry, resulting in chromosomal instability.
“I find the attempt to drive cancer cells over the edge with more chromosomal instability a little concerning because if you don’t reach the right level, it may paradoxically lead to aggressive disease,” Dr Mittal said. “Instead, our findings suggest that restoring order to cell division by targeting EZH2 can stop them from spreading.”
Linking epigenetics and metastasis
About 5 percent of cells in a TNBC primary tumour are highly likely to metastasise. These cells possess distinct features, including altered metabolism, heightened chromosomal instability and epigenetic modifications – changes to DNA or associated proteins that do not alter the genetic code directly.
Dr Mittal’s team identified EZH2 as a likely driver of metastasis in these cells. Normally, EZH2 modifies how DNA is packaged within cells. However, in cancer, EZH2 is often overproduced, silencing key genes needed for proper chromosome segregation and triggering errors during cell division.
About 5 percent of cells in a TNBC primary tumour are highly likely to metastasise.
Patient data analysis by Dr Shelley Yang Bai, Postdoctoral Associate in Cardiothoracic Surgery at Weill Cornell Medicine, revealed that higher EZH2 levels correlate with more chromosomal alterations in tumour cells. Lab experiments confirmed these findings: inhibiting EZH2 with tazemetostat, an FDA-approved drug for certain cancers, reduced chromosomal instability in TNBC cell lines, while genetically boosting EZH2 levels increased division errors.
Mouse models with elevated EZH2 exhibited more lung metastases than tumours lacking EZH2, confirming the direct link between EZH2, chromosomal instability and metastasis.
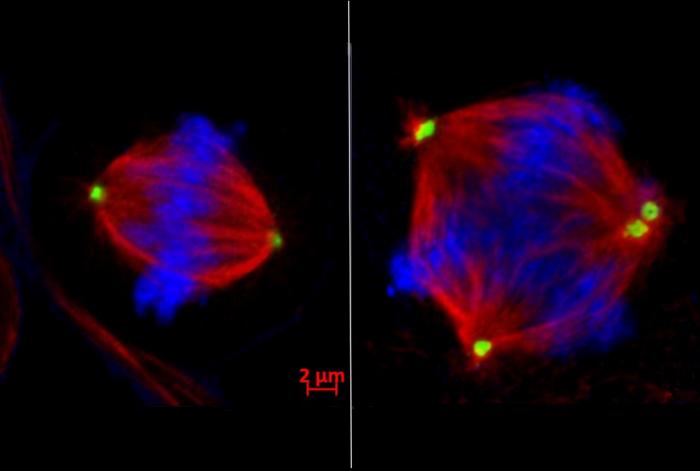

Left: Normal cell division with the chromosomes (blue) lined up and ready to be pulled into two separate daughter cells by the two centrosomes (green). Right: In faulty cell division, too many centrosomes are present, resulting in multiple cells with chromosomal instability. Credit: Dr Shelly Yang Bai
Chromosomal chaos
The research team discovered that EZH2 silences the tankyrase 1 gene, which normally ensures chromosome-separating machinery functions correctly. This silencing sets off a chain reaction: reduced tankyrase 1 levels cause an excessive buildup of CPAP – a protein that controls centrosomes. As centrosomes uncontrollably multiply, cells divide incorrectly into three or more daughter cells.
Inhibiting EZH2 restored balance in cell division and significantly reduced metastasis in preclinical models. “For the first time, we have linked EZH2, which is an epigenetic regulator, with chromosomal instability in a mechanistic fashion,” Dr Bai said.
This study provides a promising new approach to treating triple-negative breast cancer by targeting the root cause of metastases
“This study provides a promising new approach to treating triple-negative breast cancer by targeting the root cause of metastases,” said Dr Magdalena Plasilova, Associate Professor of Clinical Surgery and a surgical oncologist at New York-Presbyterian/Weill Cornell Medical Center. “I see firsthand the devastating impact of metastases on patients, and this offers hope for improved outcomes and survival rates.”
Tazemetostat could therefore potentially be repurposed as a TNBC treatment, though other drugs may offer similar or improved effects. “Our discovery opens the door for clinical trials to test EZH2 inhibitors in high-risk breast cancer and potentially other cancers that are also marked by chromosomal instability, such as lung adenocarcinoma,” said Dr Mittal.
What’s next?
By linking this enzyme to chromosomal instability, researchers have identified a promising therapeutic target that could prevent cancer from spreading. With EZH2 inhibitors like tazemetostat showing effectiveness in preclinical models, this discovery could lead to clinical trials that improve outcomes and survival for patients facing this aggressive form of breast cancer.
Related topics
Cancer research, Drug Repurposing, Enzymes, Epigenetics, Oncology, Therapeutics, Translational Science
Related conditions
triple negative breast cancer (TNBC)
Related organisations
Weill Cornell Medicine
Related people
Dr Magdalena Plasilova (Associate Professor of Clinical Surgery and a surgical oncologist at New York-Presbyterian/Weill Cornell Medical Center), Dr Shelley Yang Bai (Postdoctoral Associate in Cardiothoracic Surgery at Weill Cornell Medicine), Dr Vivek Mittal (Ford-Isom Research Professor of Cardiothoracic Surgery and member of the Sandra and Edward Meyer Cancer Center at Weill Cornell Medicine)