Human organoid research offers insights into gut cell differentiation
Posted: 3 November 2023 | Ellen Capon (Drug Target Review) | No comments yet
How stem cells become enteroendocrine cells using gut organoids could have positive implications for many gastrointestinal diseases.
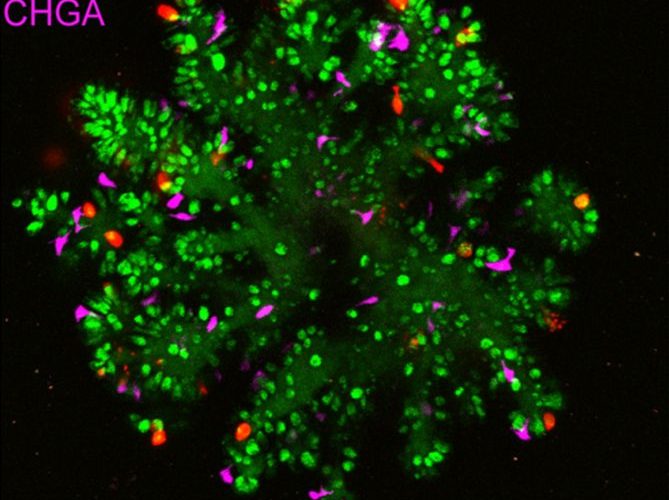

Through the process of differentiation, stem cells develop into different cell types of the human gut. Organoid group (Hubrecht Institute) researchers, collaborating with researchers at the Princess Máxima Center and Maastricht University, used gut organoids to perform a systematic CRISPR screening of 1800 human transcription factors. They identified ZNF800 as a key regulator of the differentiation of a specific gut cell type, the enteroendocrine cells. The findings may have implications for our understanding of gastrointestinal diseases and endocrine disorders, as well as pancreatic development and diabetes.
There are various cell types within the human gut which each have specific functions. Crucial cell types of the gut are enterocytes, responsible for the absorption of nutrients, goblet cells, which produce mucus, Paneth cells, which secrete antimicrobial peptides, and enteroendocrine cells (EECs), which produce various hormones. These hormones regulate digestive processes, like nutrient absorption, appetite, and glucose metabolism. In this study, Organoid group scientists investigated how stem cells become EECs using gut organoids, lab-grown miniature organs that mimic the structure and function of the real gut.
Gene regulation
The differentiation of stem cells into specific cell types happens by gene regulation, where genes in the DNA of cells are switched ‘on’ and ‘off’. Transcription factors recognise specific DNA sequences to control chromatin and transcription and form a complex system that guides the expression of the genome1, and are essential for gene regulation. First author Dr Lin Lin explained: “You can compare it to a bustling intersection where different roads lead to various cell destinies, and the vehicles on the roads symbolise different cell types. The transcription factors act as traffic lights at the intersections, determining whether cells can follow a particular direction to become specialised cells. In our study, we used CRISPR technology, a gene-editing tool, to specifically target individual transcription factors. This is like switching the ‘traffic lights’ on or off.” She continued: “By doing this, we aimed to uncover the intricate signalling system that directs cells down their predetermined routes, in the same way that traffic lights govern the movement of vehicles in a busy city.”
Master repressor
To find factors involved in stem cell differentiation, the team used CRISPR to perform a systematic screening of the entire repertoire of human transcription factors, encompassing 1800 genes. Lin said: “By screening all possible ‘traffic lights’, we identified specific ones that play a crucial role in controlling cell fate decisions. Some of these factors acted as green lights, promoting the activation of genes that guide cells towards a particular destiny, while others acted as red lights, repressing certain gene expressions and diverting cells onto different paths.”
ZNF800 was found to be a critical transcription factor in determining the balance between EECs and other cell types in the gut. “The presence of ZNF800 acts as a red light on the development of EECs: when we switched the ZNF800 ‘traffic light’ off, we saw a significant tenfold increase of EECs in the organoids. At the same time, other intestinal cell types like goblet cells and Paneth cells were suppressed,” detailed Lin.
ZNF800 also controlled the expression of other transcription factors involved in EEC differentiation, like NEUROG3, INSM1, SOX4, and PAX4. Lin noted: “The regulation of differentiation works in a hierarchical manner, where certain transcription factors act as master regulators and others as downstream effectors. We showed that ZNF800 acts as a master repressor, meaning that it influences other transcription factors and, in the end, inhibits EEC differentiation.”
Further research
This discovery may have implications for understanding gastrointestinal diseases and endocrine disorders. “Our findings provide crucial insights into the molecular mechanisms that govern cell fate decisions in the human gut, which is essential for understanding these conditions and ultimately developing treatments,” Lin said.
“Our findings provide crucial insights into the molecular mechanisms that govern cell fate decisions in the human gut, which is essential for understanding these conditions and ultimately developing treatments,”
Furthermore, it was observed that ZNF800 affected other transcription factors such as PAX4 and NEUROG3, which offers promise in diabetes research. “These transcription factors are crucial for the regulation of insulin-producing beta cells in the pancreas, raising the possibility that ZNF800 may also play a role in pancreatic development and diabetes,” concluded Lin.
The study was published in Science.
References
1 Albu M, Campitelli LF, Chen X, et al. The Human Transcription Factors. Cell [Internet]. 2018 February 8 [2023 November 1];172(4):650-65. Available from: https://doi.org/10.1016/j.cell.2018.01.029
Related topics
Organoids, Stem Cells
Related conditions
Diabetes
Related organisations
Maastricht University, Organoid group (Hubrecht Institute), Princess Máxima Center