New gene found plays essential role in stem cell differentiation
Posted: 8 December 2023 | Ellen Capon (Drug Target Review) | No comments yet
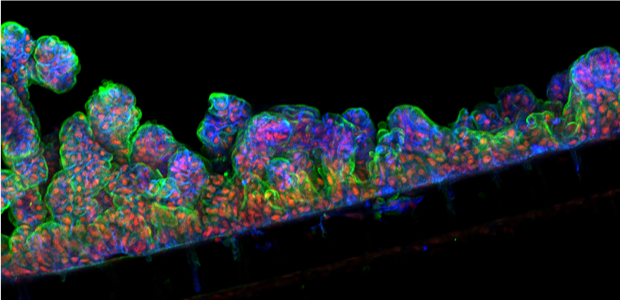
Intestinal organoids allowed researchers to understand how Rnf43 and Daam1 influence the balance of stem cell renewal and differentiation.
Researchers from the Bon-Kyoung Koo laboratory at the Institute of Molecular Biotechnology (IMBA) and the Institute for Basic Science have used intestinal organoids to find a new gene, Daam1, that plays an essential role in switching on the development of secretory cells in the intestine. Also, recent studies have reported that dishevelled-associated activator of morphogenesis 1 (DAAM1) is remarkably essential for mediating cell migration and invasion in breast cancer.1
Cells within our bodies that are damaged or dead need to be replaced to keep organs functioning. This replacement happens due to tissue-resident adult stem cells, which will only form the cell types that are found in the tissue they belong to, unlike embryonic stem cells which can form any cell type in the body. Gabriele Colozza, a postdoctoral researcher in the lab of Bon-Kyoung Koo at IMBA and now Director at the Center for Genome Engineering, Institute for Basic Science in South Korea, chose to explore how tissue-specific stem cells know which cell type to give rise to using intestinal stem cells.
Intestinal cells
Colozza said: “In our intestines, cells are exposed to extreme conditions.” Mechanical wear and tear, digestive enzymes, and varying pH values all affect intestinal cells. Therefore, stem cells in the intestine’s mucosa differentiate to form new intestinal cells. “Damaged cells have to be replaced, but it is a delicate balance between stem cell renewal and differentiation into other cell types: uncontrolled stem cell proliferation may lead to tumour formation; on the other hand, if too many stem cells differentiate, the tissue will be depleted of stem cells and ultimately unable to self-renew.”
Signalling pathways and feedback loops tune this balance, which allow cells to communicate with each other. One important pathway is called Wnt, known for its role in embryonic development. If it is left unchecked, an overactive Wnt pathway can lead to excessive cell division and the formation of tumours.
Daam1 protein
Rnf43, originally identified by Bon-Kyoung Koo, is well-understood to keep Wnt in check. Before this study, Rnf43 was known to target the Wnt receptor Frizzled and mark it for degradation. “We wanted to know how Rnf43 works, and also what – in turn – controls Rnf43 and helps it to regulate Wnt signalling.” Earlier research showed that Rnf43 on its own was not sufficient to break down the Wnt receptor Frizzled, located in the plasma membrane. “In our project, we used biochemical assays to identify which proteins interact with Rnf43.” A crucial partner of Rnf43 turned out to be the protein Daam1.
Colozza used intestinal organoids, three-dimensional (3D) cell cultures grown from adult intestinal stem cells, to investigate how Daam1 regulates Rnf43 and affects the tissues it acts in. “We found that Daam1 is required for Rnf43 to be active, so for Rnf43 to regulate Wnt signalling at all. Further work in cells showed Rnf43 needs Daam1 to move the Wnt receptor Frizzled into vesicles called endosomes. From the endosomes, Frizzled is shuttled to the lysosomes where it is degraded, dampening Wnt signalling,” Colozza explained.
“We found that when we knock-out Rnf43 or Daam1, the organoids grow into tumour-like structures.”
Creating intestinal organoids enabled the researchers to mimic the intestinal mucosa and understand how Rnf43 and Daam1 influence the balance of stem cell renewal and differentiation in the intestine. “We found that when we knock-out Rnf43 or Daam1, the organoids grow into tumour-like structures. These tumour-like organoids keep on growing, even if we withdraw the growth factors they usually depend on, such as R-spondin.”
Paneth cells
When this result was tested in mouse tissue, the scientists had a surprise discovery. “When Rnf43 was missing, the intestines grew tumours – as expected. But when Daam1 was missing, no tumours grew. We were puzzled by this striking difference: how can the loss of factors in the same pathway, that behave similarly in organoids, lead to such different outcomes?”
“When Daam1 is active, stem cells differentiate to form Paneth cells. When Daam1 is not active, the stem cells differentiate into another cell type.”
Colozza observed that intestines lacking Rnf43 were full of a type of secretory cells, Paneth cells. Contrastingly, intestines lacking Daam1contained no extra Paneth cells. Paneth cells secrete growth factors, such as Wnt, that stimulate cell division. “Daam1 is required for the efficient formation of Paneth cells. When Daam1 is active, stem cells differentiate to form Paneth cells. When Daam1 is not active, the stem cells differentiate into another cell type.”
Tumours modify their microenvironment
This link between the molecular results and Paneth cells makes clear the difference between intestines and organoids. “In organoid culture, we scientists provide growth factors, so the knockout of both Rnf43 and Daam1 lead to tumour-like organoids. But in the intestine, there is no little scientist providing growth factors. Instead, Paneth cells provide growth factors, like Wnt, and create the right conditions for stem cells to survive and divide. When Paneth cells are lacking – such as when Daam1 is not active to drive cells into becoming Paneth cells – stem cells will not divide much. But when there are too many Paneth cells – such as in intestines lacking Rnf43 – the excessive growth factors can contribute to the formation of tumours.”
This study is the first genetic proof that Daam1, a member of the non-canonical Wnt pathway, is important for specifying Paneth cells, and directly involved in the development of this crucial secretory cell. The results also shed light on the importance of stem cells. “We show that tumour cells modify their microenvironment and influence their supporting environment so that they can grow better.” This opens new opportunities in cancer research.
This was published in Science Advances.
Reference
1 Hao L, Lui Y, Mei J, et al. Overexpressed DAAM1 correlates with metastasis and predicts poor prognosis in breast cancer. Pathology – Research and Practice [Internet]. 2020 March [2023 November 27]; 216(3) Available from: https://www.sciencedirect.com/science/article/abs/pii/S0344033819319491
Related topics
Cancer research, Organoids, Stem Cells
Related conditions
Cancer
Related organisations
Institute for Basic Science (IBS), Institute of Molecular Biotechnology (IMBA)