The role of anti-viral drug development in a new pandemic era
Posted: 15 February 2024 | Dr Jay K. Varma (SIGA Technologies) | No comments yet
As humanity faces an impending era of pandemics, global collaboration among governments, organisations and industry is critical. In this article, epidemiologist Dr Jay Varma explores the urgent need for researching and developing drugs to combat a range of epidemic-prone pathogens, and reflects on the increased risk of spillovers and the challenges of vaccine limitations.


The COVID-19 pandemic serves as a reminder of the critical need for innovation in vaccine development
There is substantial evidence1 that humanity is entering a new age of pandemics. To survive this era, governments, multi-lateral organisations, foundations, and industry will need to work collectively on researching and developing new drugs that can prevent or treat a broad range of epidemic-prone pathogens, some of which have not yet been identified. This level of collaboration will require major shifts in focus and resources, drawing on the successes and failures of prior pandemic responses.
Since the 20th century, all known viral pandemics, including HIV, influenza, SARS CoV-1, SARS, CoV-1, and mpox, have resulted from a virus ‘spilling over’ from animals into humans.2 Most experts believe the frequency of such spill-overs is increasing.3 Deforestation and changes in land use have altered ecosystems,4 increasing the likelihood that humans or domestic animals will come into contact with wild animals or insects that harbour novel pathogens. Rising demand for animal protein has led to more factory farming, increasing the likelihood that diseases can spread among these animals then into humans.5 Furthermore, ever larger numbers of humans are living in cities or travelling across borders, increasing the risk of diseases being transmitted person-to-person over a long range. All of these factors are being further accelerated by climate change.6
In addition to naturally occurring threats, there is an increasing concern that future pandemics could arise from either accidental or deliberate release of pathogens. Investigations into the origins of the COVID-19 pandemic have drawn attention to the risk of researchers potentially becoming infected while working with pathogens in a laboratory7 and subsequently spreading infections in the broader community. Advances in synthetic biology have raised concern about the deliberate manipulation of pathogens8 to enhance their transmissibility and virulence for use as agents of terror or war. This is particularly worrisome for pathogens such as smallpox, which has been well documented to be manufactured as a bioweapon9 and for which population immunity is low to non-existent.
One of the most effective ways to protect humans from novel pathogens is through vaccines, therapeutics, and other medical countermeasures. In recent years, the World Health Organization (WHO), United States Centers for Disease Control and Prevention (CDC), and other leading agencies and researchers have called for new vaccines to confront these threats. The reasons for this are sound. Vaccines have been the most important technology to prevent death from infectious diseases and have been used to eradicate two dangerous viruses10 (smallpox in humans, rinderpest in animals) from the earth. Advances in molecular biology have led to highly safe, efficient, and effective platforms, such as mRNA, that can be rapidly re-purposed for different pathogens, multiple pathogens, or new strains of evolving pathogens.
Vaccines, however, do have some critical limitations. Protection from a vaccine may not be durable or complete, and persons may still require treatment with medications for their infection. Because we cannot accurately predict when or where a new pathogen will emerge, it can also be challenging to vaccinate a population at highest risk before or soon after spillover occurs. For example, scientists cannot accurately predict which town or village will have the next Nipah virus outbreak. Therefore, other than healthcare workers in areas that have previously experienced a Nipah virus outbreak, it will be difficult to accurately identify a community highly likely to benefit from a Nipah virus vaccine before an outbreak occurs. Additionally, even when a vaccine is available and an at-risk population can be identified, uptake may be minimal. Mpox, for example, is primarily circulating globally among gay and other men who have sex with men. The combination of stigma and legal prohibitions on gay sex mean many people at risk have not identified themselves as at risk and got vaccinated.11
Health agencies globally must collaborate with academic research centres and industry to address this important gap in drug treatments for pandemic-prone pathogens. The scientific challenges are enormous. Research teams must be able to rapidly identify small molecules that target biochemical pathways that are conserved across multiple viruses within a family. Unlike vaccines, however, it is far more challenging to develop a platform or backbone for (non-biologic) drugs that can be modified to cover wildly distinct pathogens.
Further, most anti-viral drugs fail clinical testing, because they must disrupt intra-cellular pathways and, therefore, have off-target activity in the host that is potentially toxic. We do have models of success in this area, however. Beginning in the late 1990s, the US Government became increasingly concerned about the threat of deliberate smallpox virus release. Funding from several US Government agencies supported private sector initiatives to identify, optimise, evaluate, and test a drug for smallpox treatment.12 That drug (tecovirimat) is now FDA-approved and stockpiled by the US and other governments for smallpox treatment,13 while also being used in clinical practice globally for treatment of mpox and other viruses within the orthopox family.
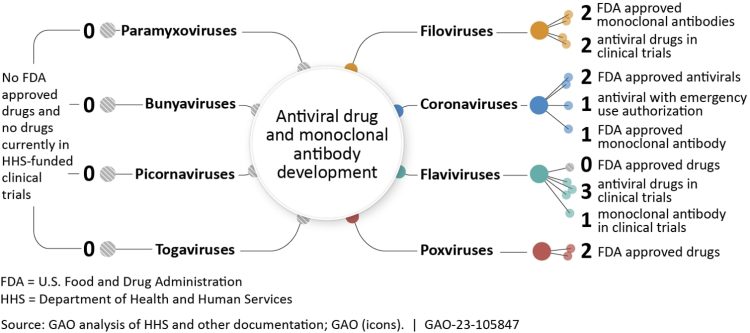

(Figure 1)
In October 2023, the US Government Accountability Office (GAO) issued several recommendations for the US Congress to accelerate development of anti-viral drugs for future pandemics.14 GAO recommends that the US government identify a single lead entity to work across the National Institutes of Health (NIH), Biomedical Advanced Research Development Agency (BARDA), CDC, Department of Defense, and other agencies to develop a priority list of viruses and viral families that present the highest risk of pandemics and for which existing countermeasures are likely insufficient. Work that is being conducted15 globally includes a focus on coronaviruses, bunyaviruses, flaviviruses, filoviruses, influenza viruses, paramyxoviruses, picornaviruses, and togaviruses (Figure 1). The lead US entity should then work across agencies to fund novel research with a goal of developing drug candidates for virus families and supporting research and development from pre-clinical through phase 1.
This support is essential if there is likely no established commercial market for that drug. New advances in artificial intelligence, organ-on-a-chip technology, and high-throughput screening will continue to make rapid development of drugs more feasible than ever before – helping to support the 100-day goal in vaccine development set by the US Government and global health agencies. At the same time, GAO recommends that Congress work with industry to identify bottlenecks in supply chains and manufacturing that are common across anti-viral drugs and support building capacity that can be used both during non-epidemic and epidemic periods.
There is now widespread consensus that the commercial anti-infective market is broken and unable to address the growing threat of antimicrobial resistance.16 To address antimicrobial resistance, various initiatives have been deployed to both ‘push’ and ‘pull’ innovation and market availability for novel drugs. In this new age of pandemics, it is important that we marshal the same level of political commitment and funding for developing and procuring drugs for pandemic-prone pathogens if we wish to save lives during future outbreaks.
About the author


Dr Jay K. Varma
Epidemiologist, Executive Vice President and Chief Medical Officer of SIGA Technologies
Dr Jay K. Varma is a leading epidemiologist and expert on the prevention and control of diseases, having led epidemic responses, developed global and national policies, and led large-scale programs in China, Southeast Asia, Africa, and the United States. From 2001-2021, he worked for the US Centers for Disease Control and Prevention with postings in Atlanta, Thailand, China, Ethiopia, and New York City. Recruited by the Mayor of New York at the peak of the COVID epidemic, Dr.Varma served from April 2020 to May 2021 as the principal scientific spokesperson and architect for New York City’s COVID-19 pandemic response. He currently serves as Chief Medical Officer at SIGA Technologies. Dr Varma graduated with highest honours from Harvard for his undergraduate education, and completed medical school, internal medicine residency, and chief residency at the University of California, San Diego School of Medicine.
- Morens D, Fauci A. Emerging Pandemic Diseases: How We Got To COVID-19. Cell. 2020 Aug;182(5).
- Preventing and Preparing for Pandemics With Zoonotic Origins. Council on Foreign Relations. Available from: https://www.cfr.org/report/preventing-and-preparing-pandemics-zoonotic-origins
- Alimi Y, et al. Report of the Scientific Task Force on Preventing Pandemics. Harvard Global Health Institute. 2021. Available from: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.hsph.harvard.edu/wp-content/uploads/sites/2343/2021/08/PreventingPandemicsAug2021.pdf
- Vora NM, Hannah L, Lieberman S, et al. Want to prevent pandemics? Stop spillovers. Nature. 2022 May 1;605(7910):419–22. Available from: https://www.nature.com/articles/d41586-022-01312-y
- Anthes E. The Risk Is Staggering, Report Says of Disease From U.S. Animal Industries. The New York Times. 2023 Jul 6; Available from: https://www.nytimes.com/2023/07/06/health/animals-agriculture-disease-spillover.html
- Mora C, McKenzie T, Gaw IM, et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nature Climate Change. 2022 Aug 8;12(12). Available from: https://www.nature.com/articles/s41558-022-01426-1.pdf
- Tufekci Z. Opinion | The Pandemic Threat That Hasn’t Gone Away. The New York Times. 2023 May 12; Available from: https://www.nytimes.com/2023/05/12/opinion/covid-lab-safety.html
- National Academies of Sciences, Engineering, and Medicine. Biodefense in the Age of Synthetic Biology. Washington, D.C.: National Academies Press; 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535877/
- Bioterrorism [Internet]. 2019. Available from: https://www.cdc.gov/smallpox/bioterrorism/public/index.html
- Orenstein WA, Ahmed R. Simply Put: Vaccination Saves Lives. Proceedings of the National Academy of Sciences. 2017 Apr 10;114(16):4031–3. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5402432/
- Stigma against gay men could worsen Congo’s biggest mpox outbreak, scientists warn. AP News. 2023 [cited 2024 Jan 22]. Available from: https://apnews.com/article/congo-monkeypox-outbreak-gay-discrimination-mpox-bdfbae117989cfa41f5e7e36d583c036?utm_source=copy&utm_medium=share
- Merchlinsky M, Albright A, Olson V, et al. The development and approval of tecoviromat (TPOXX®), the first antiviral against smallpox. Antiviral Research. 2019 Aug;168:168–74.
- Grosenbach DW, Honeychurch K, Rose EA, et al. Oral Tecovirimat for the Treatment of Smallpox. New England Journal of Medicine. 2018 Jul 5;379(1):44–53.
- Office USGA. Antiviral Drugs: Economic Incentives and Strategies for Pandemic Preparedness [Reissued with revisions on Sept. 29, 2023] | U.S. GAO. www.gao.gov. 2023. Available from: https://www.gao.gov/products/gao-23-105847
- Office USGA. Antiviral Drugs: Economic Incentives and Strategies for Pandemic Preparedness [Reissued with revisions on Sept. 29, 2023] | U.S. GAO. www.gao.gov. 2023 [cited 2024 Jan 22]. Available from: https://www.gao.gov/products/gao-23-105847
- The Antibiotic Market Is Broken—and Won’t Fix Itself [Internet]. pew.org. 2019 [cited 2024 Jan 22]. Available from: https://www.pewtrusts.org/en/about/news-room/opinion/2019/04/10/the-antibiotic-market-is-broken-and-wont-fix-itself
Related topics
Covid-19, Drug Targets, Targets, Vaccine, Vaccine development
Related conditions
Covid-19, Nipah virus (NiV), Smallpox
Related organisations
SIGA Technologies
Related people
Dr Jay K. Varma (SIGA Technologies)








