Development by design methodology: the key to successful manufacturing of patient-specific cell therapies
Posted: 11 July 2017 | Brian Hampson, David Smith | No comments yet
Recognised as the next wave of medicine, cell therapies have made significant strides over the last several years. But such advancements can present unique challenges, especially as more products move out of the clinic and toward commercialisation. And unlike typical pharmaceuticals, cell therapies rely heavily on the manufacturing process to determine their product attributes. As a result, it’s critical to ensure that cell therapy developers have the right manufacturing strategy – one that takes into account quality, cost of goods, sustainability and scalability – to ensure commercial viability.

It’s critical to ensure that cell therapy developers have the right manufacturing strategy
In general, there are two paradigms in cell therapy with substantially different implications for manufacturing: off-the-shelf and patient-specific. Off-the-shelf cell therapies are those in which one batch can be manufactured to treat multiple patients. PSCTs are those in which a separate manufacturing batch is required for each patient because the starting material is obtained either from the patient, or a donor that is immuno-matched to the patient. In manufacturing PSCTs, it is often difficult Development by design methodology: the key to successful manufacturing of patient-specific cell therapies Recognised as the next wave of medicine, cell therapies have made significant strides over the last several years. But such advancements can present unique challenges, especially as more products move out of the clinic and toward commercialisation. And unlike typical pharmaceuticals, cell therapies rely heavily on the manufacturing process to determine their product attributes. As a result, it’s critical to ensure that cell therapy developers have the right manufacturing strategy – one that takes into account quality, cost of goods, sustainability and scalability – to ensure commercial viability. to efficiently use all available manufacturing resources, such as trained staff, equipment and facilities, thus creating an additional cost of idle capacity. This can be further compounded by the uneven demand that naturally occurs for these types of therapies.
An additional challenge is that the distribution of PSCTs can be expensive, with common hurdles such as transport time restrictions because of the short shelf life of fresh products, or the added shipment requirements for cryopreserved productions.
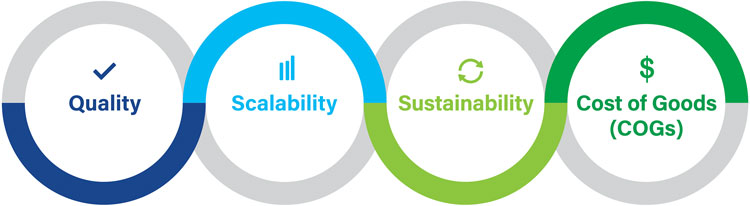

The four areas of Development by Design
Development by Design
To address these unique challenges, we recommend applying a structured approach that we call Development by Design (DbD) – a commercial manufacturing strategy that considers quality, scalability, sustainability, and cost of goods sold (COGS). Working through these four areas of DbD at an early stage in the development of a cell therapy can help avoid costly, difficult, and time-consuming manufacturing changes later by providing a prioritised roadmap of what needs to be addressed and when. DbD expands on a Quality by Design approach, which formalises product design, automates manual testing, and streamlines troubleshooting, by considering three additional drivers.
Quality is a key concern for any drug developer. However, for cell therapy, where there is heavy reliance on process to meet critical quality attributes (CQA) of the final product – the manual, open and human-dependent nature of many of the process steps presents significant risk.
With PSCTs, where there is only one patient per lot of drug produced, the strength of the process is directly related to reducing the failure of treating the patient. In addition, it is often impractical to perform the complete range of tests that are typically required to confirm all the CQAs have been met for each lot. As a result, a robust process with validated ability to manufacture high-quality products consistently is critical.
For the manufacture of cell therapies, retention of cell function and quality is of the utmost importance in order to preserve product efficacy, as the cell is the product of interest.
Scalability
Scalability is one of the most unique challenges to PSCTs. Developers may come up with an innovative cell therapy and guide the product through all the stages of clinical development, only to discover that once they reach the commercialisation stage, the process is not scalable.
For a typical pharmaceutical process, once a product has been established, the process is passed to the manufacturing engineers who then find a way to increase scale for commercialisation. However, PSCTs offer a new challenge for process scalability, where the manufacturing process must be scaled out, to continue producing one batch for each patient (as opposed to scale up, in traditional pharmaceuticals, in which a larger batch of the product is made for many patients). Scale-out can be achieved by advances in engineering and manufacturing technology, reducing the number of complex, labour-intensive and open-process steps that are commonplace in cell therapy production.
To ensure scalability, manufacturers should not only start planning for their desired future state early in the product development cycle, but also understand how scalability can be achieved for their particular process and minimise cost per dose as product rate increases. Furthermore, developers should work closely with their manufacturing partners to leverage their knowledge and expertise to ensure that the process, including supply chain and logistics, are scalable and will be commercially viable for the future.
Moving a process from clinical scale, in which tens of hundreds of patient doses are made each year, to a commercial-scale process in which thousands to tens of thousands of doses are manufactured, can present comparability risks. Cell therapy products are highly complex, with one or more mechanisms of action that are often not understood. In addition, there is currently a lack of analytical tools and in vivo models to determine product comparability.
Comparability is a risk across any of the DbD elements – it is recognising the fact that if cell developers wait until later to address quality, COGS, scale or sustainability, major changes might need to be made, and once these changes are made there is the need to demonstrate that the post-change product is comparable to the pre-change product. This can be very costly and time consuming and, in the worst case, may require additional clinical testing to show comparable clinical outcome.
Sustainability
For PSCTs, there is a very real risk that manufacturing cannot be sustained over the full product life cycle. For example, a major risk is disruption in the relatively fragile and immature supply chain, which could halt manufacturing. In the worst case, a process step relies on supply chain elements that become unavailable, requiring changes to be developed, tested and comparability demonstrated. To mitigate risks to sustainability, companies need to assess the full range of supply chain inputs to the manufacturing process, including reagents, consumables, equipment and human resources.
Cell therapy developers should be mindful of the market and build good relationships with multiple suppliers
Cell therapy requires ingredients that are of the highest quality and are tightly regulated, are rarely off the shelf, and often available from only one supplier. Therefore, they are very expensive. Procuring high-quality ingredients is the first hurdle a cell therapy developer faces. Currently, there are few providers who can provide products that will meet GMP standards. Market demand can also cause shortages. Given that few direct actions can be taken to mitigate this risk, cell therapy developers should be mindful of the market and build good relationships with multiple suppliers. Hopefully, as the cell therapy market grows, more and more suppliers will be willing to invest in the quality systems required.
Cost of goods sold
For a cell developer, considering COGs early in the manufacturing process is vital to a successful launch, especially when scale is set to greatly increase.
COGs for cell therapies can be divided into several categories:
- Direct costs (directly mapped to the patient-specific lot being manufactured)
- Labour – production, testing, quality assurance, materials handling
- Materials – production, testing
- Third-party services – outplant testing, shipping, cell collection, irradiation, etc
- Indirect/overhead costs
- Supervision, management
- Quality events (eg, process deviations, out-of-specification events)
- Facility operations – cleanroom operations, maintenance/repair, utilities, rent
- Materials and outside services management
- Sustaining technical support
- Amortisation of non-recurring investment
- Non-recurring design, development, engineering
- Capital expenditure – equipment, facilities
- Absorption of cost of failed product lots.
Compared to traditional biologics, managing COGs for PSCTs presents a variety of unique challenges. First, PSCTs must be manufactured one at a time, which limits the use of traditional economies of scale for cost savings. Instead, COGs must be reduced through methods such as technology optimisation, including automation and integration of manufacturing steps and reduced costs of human labour. Second, consistently utilising all available manufacturing resources (eg, trained staff, equipment, facilities), can often be difficult, creating an additional cost of idle capacity. Third, the distribution of PSCTs can be expensive, with common hurdles including transport time restrictions due to the short shelf life of fresh products, or the added shipment requirements for cryopreserved products.
To reduce COGs for PSCTs, companies must consider their target commercial COGs early in product development. It is imperative to keep this top of mind in all major decisions – from considering whether to manufacture in house or use a contract manufacturer, to determining unit operations that can be automated, the comparability risk of changing manufacturing processes in late-phase development, and so on.
Depending on the volume of production that is expected once commercialised, investment in automation prior to commercialisation may have a significant long-term effect on the reduction of direct costs. Without undergoing COGs analysis early in development, a PSCT developer cannot be certain that their manufacturing process is optimised for success. Fortunately, with such an analysis, the developer has a solid roadmap for long-term commercial viability.
Planning ahead is crucial
As cell therapy developers move from one clinical phase to the next, it is critical to avoid the tunnel vision of focusing only on successfully completing a trial. It is equally important to consider long-term goals. One way to do this is to create a manufacturing strategy that addresses the elements of DbD – quality, cost, scalability and sustainability.
Considering DbD early in the development of a cell therapy does not necessarily mean that a large investment needs to be made earlier in the process. However, it does mean cell therapy developers need to plan ahead. Working through these four areas can mean the difference between a product that is ready for commercial success versus one that needs significant additional investment of time and money for commercial viability.
Biographies




Related topics
Drug Development, Stem Cells
Related organisations
PCT
Related people
Brian Hampson, David Smith








