Scientists have identified novel molecular biomarkers in cells that spread triple-negative breast cancer
Posted: 9 August 2022 | Ria Kakkad (Drug Target Review) | No comments yet
The researchers identified key molecular differences between triple-negative breast cancer cells that cling to an initial tumour and those that venture off to form distant tumours.

In their research of triple-negative breast cancer, scientists at Johns Hopkins Medicine, US have identified key molecular differences between cancer cells that cling to an initial tumour and those that venture off to form distant tumours. The findings, which were recently published in Science Translational Medicine, could pave way for developing new treatments that target these molecular variations.
The lethal nature of this type of cancer is marked by the fact that its cells lack molecular flags on its surface that connect with the hormone’s oestrogen and progesterone and a cancer growth-promoting protein called Her2-neu. Many current breast cancer therapies work by targeting those flags, rendering them of little use to those with triple-negative tumours.
The team looked at molecular differences between initial, or primary, triple-negative breast cancer sites and areas where it spread, or metastatic sites, among three different types of cells: mouse models, human cancers implanted into mice and samples of both primary and metastatic tissues.
The researchers used a combination of machine learning, cellular imaging, and biochemical analysis to identify differences in the genetic expression patters of initial and metastatic tumours.
The team found several unique properties in the cells of mice engineered to have the mouse-version of triple-negative breast cancers, and mice implanted with tumours from people with triple-negative breast cancer.
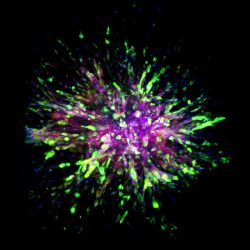

A triple-negative breast cancer organoid invades collagen tissue
[Credit: Eloïse Grasset, Johns Hopkins Medicine].
In addition, the scientists discovered that when triple-negative breast cancer cells invade other tissues on their way to another part of the body, they gain two cellular properties: better movement and survival.
To do this, breast cancer cells gain a cellular skeleton protein dubbed vimentin, which enhances the migratory ability of so-called mesenchymal cells, a cell type typically found in bones and bone marrow that moves around and makes new cells.
Triple-negative breast cancer cells also gain survival advantages by producing a protein called E-cadherin, typically found in epithelial cells that line the ducts and coverings of organs and frequently renew themselves.
When triple-negative breast cancer cells gain such survival and migratory qualities, scientists classify their cellular state as so-called hybrid epithelial mesenchymal (EMT) cells.
To look more closely at molecules involved in hybrid EMT states, the team used machine learning techniques to find patterns among each cell’s expression of RNA, a cousin of DNA involved in protein production. The scientists found that most of the metastatic cells morph into the more mobile, better surviving, hybrid EMT state. They then validated these states in samples from eight patients with triple-negative tumours, examining both primary tumours and tissues from metastatic sites of the same patients.
At the molecular level, the most metastatic cells produced five proteins called transcription factors (Grhl2, Foxc2, Zeb1, Zeb2 and Ovol1) that foster the making of proteins involved in either cancer cell invasion or colony formation.
The team are now studying ways to block the transcription factors’ genes or their resulting proteins to halt metastatic cancer growth as well as whether the same molecular and cellular changes happen in other cancers, such as those in the colon, adrenal glands, stomach, and small intestine.
Related topics
Biomarkers, Molecular Biology, Molecular Modelling, Oncology
Related conditions
Triple-negative breast cancer
Related organisations
Johns Hopkins Medicine