Utilising CAAR T cells to treat antibody-caused brain disease
Posted: 6 November 2023 | Drug Target Review | No comments yet
Researchers have engineered CAAR T cells to destroy harmful antibodies, improving NMDA receptor encephalitis treatment.
A new treatment for NMDA receptor encephalitis, the commonest form of antibody-caused brain disease which about 200 to 300 people are estimated to develop in Germany per year, has been discovered by researchers at DZNE and Charité – Universitätsmedizin Berlin. The team engineered specialised chimeric autoantibody receptor T cells (CAAR-T cells), designed for injection into patients, to identify and destroy the cells responsible for producing harmful antibodies.
In mouse models, this method has already demonstrated its accuracy. Professor Harald Prüss, who conducts research at DZNE’s Berlin site and at Charité – Universitätsmedizin Berlin, said: “In pre-clinical testing, we have succeeded in switching off selectively the cells in which these misdirected antibodies are formed.”
Targeted approach
NMDA receptor encephalitis has severe symptoms, such as memory impairment, epileptic seizures, impaired consciousness, and psychosis. Severe cases may even need treatment in an intensive care unit, so this new procedure would be a substantial improvement over current therapy.
Prüss explained: “Instead of suppressing the entire immune system and eliminating not only the misdirected antibodies, but also the more than 99 percent of beneficial antibodies, as we have done in the past, we set out to find a targeted approach.”
The disease causes the immune system to attack the patients’ own body. Many antibodies designed to eliminate viruses or bacteria are in human blood, but problems occur when some of these antibodies mistakenly target the body’s own tissues. If they cross the so-called blood-brain barrier, they can attack the brain, and it understood that there is a whole variety of “autoimmune encephalopathies,” each triggered by different antibodies.
CAAR T cells
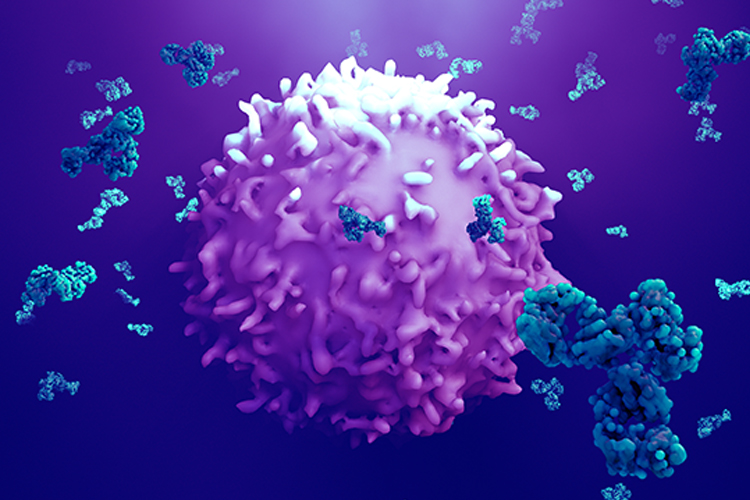
The scientists developed a detailed procedure for the new, targeted therapy. Dr Momsen Reincke, who researches at DZNE and Charité, explained: “We use human T cells, which we can obtain from patients’ blood, and modify them by adding a coupling molecule.” This genetic reprogramming changes the T cells into CAAR T cells. When reintroduced into the body, the CAAR T cells specifically target those body cells that produce the misdirected antibodies. “The surface of these cells is shaped in such a way that the CAAR T cells precisely dock onto them and kill them.” Notably, cells producing other antibodies which therefore have a different surface, remain untouched.
The term “chimera” within the acronym CAAR refers to the principle of artificially constructing a molecule from different components. Cancer therapy already has a treatment that follows a similar pattern, but the Berlin researchers are the first to successfully apply this concept to an autoimmune disease of the brain.
Promising future
The researchers hope to test this new therapy in a human clinical trial, which could begin in one to two years, according to current estimates. Pruss said: “In the beginning, we will take a blood sample from every affected person in order to obtain T cells from them individually.” He continued: “Given the rapid developments in the field of cell therapies, however, the next step could presumably be to use cells with which treatment no longer has to be patient specific. That would be far less costly.”
Ideally, a single application of the reprogrammed cells could definitively cure this type of autoimmune encephalitis, as current knowledge suggests that once killed, the cells that make the problematic antibodies typically do not reproduce.
The method could be adapted to work in other autoimmune encephalopathies and not only in the NMDA receptor variant.
The study was published in Cell.
Related topics
Antibodies, Chimeric Antigen Receptors (CARs), Immunology, Neurosciences
Related conditions
NMDA receptor encephalitis
Related organisations
Charité Universitätsmedizin Berlin, German Center for Neurodegenerative Diseases (DZNE)